Pharmacokinetics and haemodynamics of candesartan cilexetil in hypertensive patients on regular haemodialysis
- PMID: 10383542
- PMCID: PMC2014260
- DOI: 10.1046/j.1365-2125.1999.00939.x
Pharmacokinetics and haemodynamics of candesartan cilexetil in hypertensive patients on regular haemodialysis
Abstract
Aims: The pharmacokinetic profile of candesartan cilexetil might be altered in patients with end-stage renal disease (ESRD). No data are available about the pharmacokinetics and haemodynamics of the angiotensin II receptor antagonist candesartan cilexetil in ESRD patients on regular haemodialysis (HD).
Methods: We performed a repeated dose study (8 mg candesartan cilexetil once daily) in eight male HD patients over a treatment period of 5 days with an additional observation period of 3 days.
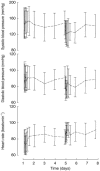
Results: Pharmacokinetic analysis with nonlinear mixed effects modeling (NONMEM) over the whole treatment period revealed a dependency of the volume of distribution on body weight and of the metabolic clearance on age and body weight in the studied population. No significant drug elimination by HD was observed. The estimated metabolic and intercompartmental clearances were 83 ml min-1 (CV 39%) and 9.9 ml min-1, respectively. The unexplained random variability of the final two compartment model was 30%. In one patient with adult polycystic kidney disease oral clearance decreased during the observation period, attributable to a significant increase in bioavailability. Maximum observed changes in blood pressure were -50/-27+/-14/8 mmHg on day 5 with haemodialysis therapy as compared with changes in blood pressure of -14/-12+/-14/8 mmHg on day 1 without haemodialysis treatment. The observed maximum decrease in systolic blood pressure correlated with the amount of ultrafiltration during the HD session on day 5 (r=0.70, P<0.05). In two patients, one of whom was binephrectomized, severe hypotensive episodes were observed during this HD session.
Conclusions: HD does not influence the elimination kinetics of candesartan. The observed inter- and intraindividual variability of oral clearance and the pronounced influence of HD-induced volume contraction on the haemodynamic effects of candesartan makes it mandatory to carefully monitor HD patients treated with candesartan cilexetil.
Figures
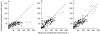
References
-
- Kubo K, Kohara Y, Imamiya E, et al. Nonpeptide angiotensin II receptor antagonists. Synthesis and biological activity of benzimidazolecarboxylic acids. J Med Chem. 1993;36:2182–2195. - PubMed
-
- Kubo K, Kohara Y, et al. Nonpeptide angiotensin II receptor antagonists. Synthesis and biological activity of potential prodrugs of benzimidazole-7-carboxylic acids. J Med Chem. 1993;36:2343–2349. - PubMed
-
- Morimoto S, Ogihara T. TCV-116: a new angiotensin II type-1 antagonist Cardiovasc. Drug Rev. 1994;12:153–164.
-
- Shibouta Y, Inada Y, Ojima M, et al. Pharmacological profile of a highly potent and long-acting angiotensin II receptor antagonist, 2-ethoxy-1-[[2′-(1H-tetrazol-5-yl)biphenyl-4-yl]-methyl]-1H-benzimidazole-7-carboxylic acid (CV-11974) and its prodrug (±)-1-(cyclohexyloxycarbonyloxy)-ethyl-2-ethoxy-1-[[2′-(1H-tetrazol-5-yl)biphenyl-4-yl]-methyl]-1H-benzimidazole-7-carboxylate (TCV-116) J Pharmacol Exp Ther. 1993;266:114–120. - PubMed
-
- Kondo T, Yoshida K, Yoshimura Y, Motohashi M, Tanayama S. Disposition of the new angiotensin II receptor antagonist candesartan cilexetil in rats and dogs. Drug Res. 1996;46:600. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

