A prospective study of adverse drug reactions in hospitalized children
- PMID: 10383547
- PMCID: PMC2014265
- DOI: 10.1046/j.1365-2125.1999.00943.x
A prospective study of adverse drug reactions in hospitalized children
Abstract
Aims: There are few publications of adverse drug reactions (ADRs) among paediatric patients, though ADR incidence is usually stated to be higher during the first year of life and in male patients. We have carried out a prospective study to assess the extent, pattern and profile risk for ADRs in hospitalized patients between 1 and 24 months of age.
Methods: An intensive events monitoring scheme was used. A total of 512 successive admissions to two medical paediatric wards (47 beds) were analysed. The hospital records were screened daily during two periods (summer, 105 days and winter, 99 days), and adverse clinical events observed were recorded.
Results: A total of 282 events were detected; of these, 112 were considered to be manifestations of ADRs. The cumulative incidence was 16.6%, no differences being observed between periods. Although there were no differences between patients under and over 12 months of age, risk was found to be significantly higher among girls compared with boys (RR=1.66, 95% CI 1.03-2.52). The gastro-intestinal system was most frequently affected. The therapeutic group most commonly implicated was anti-infective drugs and vaccines (41.5%). The ADRs were mild or moderate in over 90% of cases. A consistent relationship was noted between the number of drugs administered and the incidence of ADRs.
Conclusions: Hospitalized patients exhibited an ADR risk profile that included female sex and the number of drugs administered. No particular age predisposition was observed. The most commonly prescribed drugs are those most often implicated in ADRs in paediatric patients.
Figures
 winter). Note that a single admission can have several clinical manifestations.
winter). Note that a single admission can have several clinical manifestations.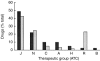
 winter). Note that a single admission can have several suspected drugs. J = anti-infective agents, including vaccines; N = central nervous system, including analgesics; C = cardiovascular; A = digestive system, including vitamins; H = hormones; R = respiratory system; B = blood and haematopoietic organs
winter). Note that a single admission can have several suspected drugs. J = anti-infective agents, including vaccines; N = central nervous system, including analgesics; C = cardiovascular; A = digestive system, including vitamins; H = hormones; R = respiratory system; B = blood and haematopoietic organs

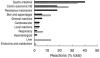
 ) in suspected adverse drug reactions for each therapeutic group. J = anti-infective agents, including vaccines; N = central nervous system, including analgesics; R = respiratory system; A = digestive system, including vitamins; H = hormones; C = cardiovascular; B = blood and haematopoietic organs; D = dermatologic; S = sensory organs; G = genitourinary therapy, including sex hormones; V = Various; P = antiparasitic drugs.
) in suspected adverse drug reactions for each therapeutic group. J = anti-infective agents, including vaccines; N = central nervous system, including analgesics; R = respiratory system; A = digestive system, including vitamins; H = hormones; C = cardiovascular; B = blood and haematopoietic organs; D = dermatologic; S = sensory organs; G = genitourinary therapy, including sex hormones; V = Various; P = antiparasitic drugs.References
-
- Olive G. Pharmacovigilance chez l’enfant. Therapie. 1989;44:141–144. - PubMed
-
- Meyboom RHB. Adverse reactions to drugs in children, experiences with ‘spontaneous monitoring’ in the Netherlands. Bratsl Lek Listy. 1991;92:554–559. - PubMed
-
- McQueen AG. Pharmacological basis of adverse drug reactions. In: Speight TM, editor. Avery’s Drug Treatment. 3. Auckland: Adis Press; 1987. pp. 223–254.
-
- Leary PM. Adverse reactions in children. Special considerations in prevention and management. Drug Safety. 1991;6:171–182. - PubMed
-
- Capellá D, Avila P, Cabeza L, Moreno V, Vidal X, Laporte JR. Cuatro años de experiencia en farmacovigilancia. Med Clin (Barc) 1988;91:93–96. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

