The Nf1 tumor suppressor regulates mouse skin wound healing, fibroblast proliferation, and collagen deposited by fibroblasts
- PMID: 10383727
- PMCID: PMC2854506
- DOI: 10.1046/j.1523-1747.1999.00609.x
The Nf1 tumor suppressor regulates mouse skin wound healing, fibroblast proliferation, and collagen deposited by fibroblasts
Abstract
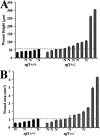
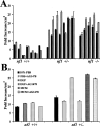
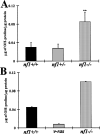
Neurofibromatosis type 1 patients develop peripheral nerve tumors (neurofibromas) composed mainly of Schwann cells and fibroblasts, in an abundant collagen matrix produced by fibroblasts. Trauma has been proposed to trigger neurofibroma formation. To test if loss of the neurofibromatosis type 1 gene (Nf1) compromises fibroblast function in vivo following trauma, skin wounding was performed in Nf1 knockout mice. The pattern and amount of collagen-rich granulation bed tissue, manufactured by fibroblasts, was grossly abnormal in 60% of Nf1+/- wounds. Nf1 mutant fibroblasts showed cell autonomous abnormalities in collagen deposition in vitro that were not mimicked by Ras activation in fibroblasts, even though some Nf1 effects are mediated through Ras. Nf1+/- skin wound fibroblasts also proliferated past the normal wound maturation phase; this in vivo effect was potentiated by muscle injury. In vitro, Nf1+/- fibroblasts showed higher proliferation in 10% serum than Nf1+/+ fibroblasts. Macrophage-conditioned media or epidermal growth factor potentiated Nf1+/- fibroblast proliferation in vitro, demonstrating abnormal response of mutant fibroblasts to wound cytokines. Thus Nf1 is a key regulator of fibroblast responses to injury, and Nf1 mutation in mouse fibroblasts causes abnormalities characteristic of human neurofibromas.
Figures



References
-
- Basu TN, Gutmann DH, Fletcher JA, Glover TW, Collins FS, Downward J. Aberrant regulation of ras proteins in malignant tumour cells from type 1 neurofibromatosis patients. Nature. 1992;356:713–715. - PubMed
-
- Berg RA. Determination of 3- and 4-hydroxyproline. Methods Enzymol. 1982;82:372–398. - PubMed
-
- Brannan CI, Perkins AS, Vogel KS, et al. Targeted disruption of the neurofibromatosis type-1 gene leads to developmental abnormalities in heart and various neural crest-derived tissues. Genes Dev. 1994;8:1019–1029. - PubMed
-
- Brown RL, Breeden MP, Greenhalgh DG. PDW and TGFalpha act synergistically to improve wound healing in genetically diabetic mouse. J Surg Res. 1994;56:562–570. - PubMed
-
- Clark RA. Mechanisms of cutaneous wound repair. In: Fitzpatrick TB, Eisen AZ, Wolff K, Freedberg IM, Austen KF, editors. Dermatology in General Medicine. McGraw-Hill; New York: 1993. pp. 473–486.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

