How photosynthetic bacteria harvest solar energy
- PMID: 10383951
- PMCID: PMC93873
- DOI: 10.1128/JB.181.13.3869-3879.1999
How photosynthetic bacteria harvest solar energy
Figures
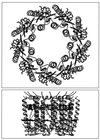
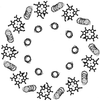
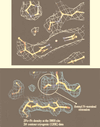
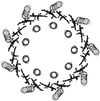

References
-
- Aagaard J, Sistrom W R. Control of the synthesis of reaction centre bacteriochlorophylls in photosynthetic bacteria. Photochem Photobiol. 1972;15:209–225. - PubMed
-
- Barz W P, Francia F, Venturoli G, Melandri B A, Verméglio A, Oesterhelt D. Role of PufX protein in photosynthetic growth of Rhodobacter sphaeroides. 1. PufX is required for efficient light-driven electron transfer and photophosphorylation under anaerobic conditions. Biochemistry. 1995;34:15235–15247. - PubMed
-
- Barz W P, Verméglio A, Francia F, Verturoli G, Melandri B A, Oesterhelt D. Role of PufX protein in photosynthetic growth of Rhodobacter sphaeroides. 2. PufX is required of efficient ubiquinone/ubiquinol exchange between the reaction centre Q(B) site and the cytochrome b/c1complex. Biochemistry. 1995;34:15248–15258. - PubMed
-
- Bauer C E, Bird T H. Regulatory circuits controlling photosynthesis gene expression. Cell. 1996;85:5–8. - PubMed
-
- Blankenship R E, Madigan M T, Bauer C E. Anoxygenic photosynthetic bacteria. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

