Characterization of the major superoxide dismutase of Staphylococcus aureus and its role in starvation survival, stress resistance, and pathogenicity
- PMID: 10383955
- PMCID: PMC93877
- DOI: 10.1128/JB.181.13.3898-3903.1999
Characterization of the major superoxide dismutase of Staphylococcus aureus and its role in starvation survival, stress resistance, and pathogenicity
Abstract
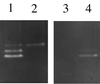
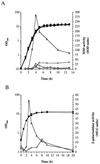
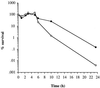
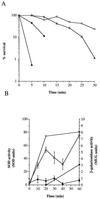
A Staphylococcus aureus mutant (SPW1) which is unable to survive long-term starvation was shown to have a transposon insertion within a gene homologous to the sodA family of manganese-dependent superoxide dismutases (SOD). Whole-cell lysates of the parental 8325-4 strain demonstrated three zones of SOD activity by nondenaturing gel electrophoresis. The activities of two of these zones were dependent on manganese for activity and were absent in SPW1. The levels of SOD activity and sodA expression were growth-phase dependent, occurring most during postexponential phase. This response was also dependent on the level of aeration of the culture, with highest activity and expression occurring only under high aeration. Expression of sodA and, consequently, SOD activity could be induced by methyl viologen but only during the transition from exponential- to postexponential-phase growth. SPW1 was less able to survive amino acid limitation and acid stress but showed no alteration in pathogenicity in a mouse abscess model of infection compared to the parental strain 8325-4.
Figures


References
-
- Battistoni A, Donnarumma G, Greco R, Valenti P, Rotilio G. Overexpression of a hydrogen peroxide-resistant periplasmic Cu, Zn superoxide dismutase protects Escherichia coli from macrophage killing. Biochem Biophys Res Commun. 1998;243:804–807. - PubMed
-
- Beaman L, Beaman B L. The role of oxygen and its derivatives in microbial pathogenesis. Annu Rev Microbiol. 1984;38:27–48. - PubMed
-
- Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971;44:276–287. - PubMed
-
- Benov L T, Fridovich I. Escherichia coli expresses a copper- and zinc-containing superoxide dismutase. J Biol Chem. 1994;269:25310–25314. - PubMed
-
- Benov L, Fridovich I. A superoxide dismutase mimic protects sodA sodB Escherichia coli against aerobic heating and stationary-phase death. Arch Biochem Biophys. 1995;332:291–294. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

