Effects of mitoxantrone on action potential and membrane currents in isolated cardiac myocytes
- PMID: 10385229
- PMCID: PMC1566021
- DOI: 10.1038/sj.bjp.0702547
Effects of mitoxantrone on action potential and membrane currents in isolated cardiac myocytes
Abstract
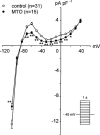
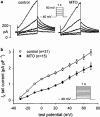
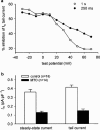
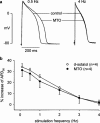
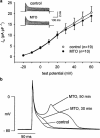
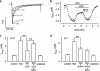
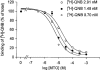
1. The effects of mitoxantrone (MTO), an anticancer drug, on the membrane electrical properties of cardiac myocytes were investigated using the whole-cell clamp technique. 2. In isolated guinea-pig ventricular myocytes, 30 microM MTO induced a time-dependent prolongation of action potential duration (APD) which was occasionally accompanied by early afterdepolarizations. APD prolongation was preserved in the presence of 10 microM tetrodotoxin and showed reverse rate-dependence. 3. Both the inward rectifier K+ current (I(KI)) and the delayed rectifier K+ current (I(K)) of guinea-pig ventricular myocytes were significantly depressed by 30 microM MTO. The rapidly activating component of I(k) (I(Kr)) seemed to be preferentially blocked by MTO. The transient outward current was not affected by MTO in rat ventricular myocytes. 4. Thirty microM MTO had no direct effect on the L-type Ca2+ current (I(Ca(L))), but reversed the inhibitory effect of 1 microM carbamylcholine but not the A1-adenosine receptor agonist (-)-N6-phenylisopropyladenosine (1 microM) on I(Ca(L)) enhanced by 50 nM isoprenaline in guinea-pig ventricular myocytes. In guinea-pig atrial mycotyes, 30 microM MTO inhibited by 93% the muscarinic receptor gated K+ current (I(K,ACh)) evoked by 1 microM carbamylcholine, whereas I(K,ACh) elicited by 100 microM GTPgammaS, a nonhydrolysable GTP analogue, was only decreased by 12%. 5. The specific binding of [3H]QNB, a muscarinic receptor ligand, to human atrial membranes was concentration-dependently displaced by MTO (1-1000 microM). 6. In conclusion, MTO blocks cardiac muscarinic receptors and prolongs APD by inhibition of I(KI) and I(Kr). The occasionally observed early afterdepolarizations may signify a potential cardiac hazard of the drug.
Figures
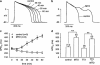

Similar articles
-
Inhibitory effects of JTV-519, a novel cardioprotective drug, on potassium currents and experimental atrial fibrillation in guinea-pig hearts.Br J Pharmacol. 2000 Dec;131(7):1363-72. doi: 10.1038/sj.bjp.0703713. Br J Pharmacol. 2000. PMID: 11090108 Free PMC article.
-
Effects of mitoxantrone on excitation-contraction coupling in guinea pig ventricular myocytes.J Pharmacol Exp Ther. 2000 May;293(2):501-8. J Pharmacol Exp Ther. 2000. PMID: 10773021
-
Inhibitory effects of aprindine on the delayed rectifier K+ current and the muscarinic acetylcholine receptor-operated K+ current in guinea-pig atrial cells.Br J Pharmacol. 1999 Feb;126(3):751-61. doi: 10.1038/sj.bjp.0702334. Br J Pharmacol. 1999. PMID: 10188988 Free PMC article.
-
Effects of azimilide on the muscarinic acetylcholine receptor-operated K+ current and experimental atrial fibrillation in guinea-pig hearts.J Pharmacol Sci. 2007 Nov;105(3):229-39. doi: 10.1254/jphs.fp0070940. Epub 2007 Oct 27. J Pharmacol Sci. 2007. PMID: 17965539
-
Ionic mechanisms mediating the differential effects of methohexital and thiopental on action potential duration in guinea pig and rabbit isolated ventricular myocytes.Anesthesiology. 1999 Jan;90(1):156-64. doi: 10.1097/00000542-199901000-00021. Anesthesiology. 1999. PMID: 9915324
References
-
- ABI-GERGES N., ESCHENHAGEN T., HOVE-MADSEN L., FISCHMEISTER R., MÉRY P.-F. Methylene blue is a muscarinic antagonist in cardiac myocytes. Mol. Pharmacol. 1997;52:482–490. - PubMed
-
- ALDERTON P.M., GROSS J., GREEN M.D. Comparative study of doxorubicin, mitoxantrone, and epirubicin in combination with ICRF-187 (ADR-529) in chronic cardiotoxicity animal model. Cancer Res. 1992;52:194–201. - PubMed
-
- BACKX P.H., MARBAN E. Background potassium current active during the plateau of the action potential in guinea pig ventricular myocytes. Circ. Res. 1993;72:890–900. - PubMed
-
- BELARDINELLI L., LINDEN J., BERNE R.M. The carciac effects of adenosine. Prog. Cardiovasc. Dis. 1989;32:73–97. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

