Parallel modulation of receptor for activated C kinase 1 and protein kinase C-alpha and beta isoforms in brains of morphine-treated rats
- PMID: 10385232
- PMCID: PMC1566027
- DOI: 10.1038/sj.bjp.0702555
Parallel modulation of receptor for activated C kinase 1 and protein kinase C-alpha and beta isoforms in brains of morphine-treated rats
Abstract
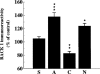
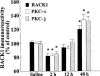
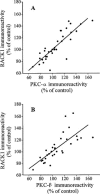
1. Receptor for activated C kinase 1 (RACK1) is an intracellular receptor for protein kinase C (PKC) that regulates the cellular enzyme localization. Because opiate drugs modulate the levels of brain PKC (Ventayol et al., 1997), the aim of this study was to assess in parallel the effects of morphine on RACK1 and PKC-alpha and beta isozymes densities in rat brain frontal cortex by immunoblot assays. 2. Acute morphine (30 mg kg(-1), i.p., 2 h) induced significant increases in the densities of RACK1 (33%), PKC-alpha (35%) and PKC-beta (23%). In contrast, chronic morphine (10-100 mg kg(-1), i.p., 5 days) induced a decrease in RACK1 levels (22%), paralleled by decreases in the levels of PKC-alpha (16%) and PKC-beta (16%). 3. Spontaneous (48 h) and naloxone (2 mg kg(-1), i.p., 2 h)-precipitated morphine withdrawal after chronic morphine induced marked up-regulations in the levels of RACK1 (38-41%), PKC-alpha (51-52%) and PKC-beta (48-62%). 4. In the same brains and for all combined treatments, there were significant positive correlations between the density of RACK1 and those of PKC-alpha (r=0.85, n = 35) and PKC-beta (r=0.75, n=32). 5. These data indicate that RACK1 is involved in the short- and long-term effects of morphine and in opiate withdrawal, and that RACK1 modulation by morphine or its withdrawal is parallel to those of PKC-alpha and beta isozymes. Since RACK1 facilitates the PKC substrate accessibility, driving its cellular localization, the coordinate regulation of the PKC/RACK system by morphine could be a relevant molecular mechanism in opiate addiction.
Figures


References
-
- AMMER H., SCHULZ R. Enhanced stimulatory adenylyl cyclase signaling during opioid dependence is associated with a reduction in palmitoylated Gsα. Mol. Pharmacol. 1997;52:993–999. - PubMed
-
- BEITNER-JOHNSON D., GUITART X., NESTLER E.J. Glial fibrillary acidic protein and the mesolimbic dopamine system: regulation by chronic morphine and Lewis-Fischer strain differences in the rat ventral tegmental area. J. Neurochem. 1993;61:1766–1773. - PubMed
-
- BUSQUETS X., ESCRIBÁ P.V., SASTRE M., GARCÍA-SEVILLA J.A. Loss of protein kinase C-αβ in brain of heroin addicts and morphine-dependent rats. J. Neurochem. 1995;64:247–252. - PubMed
-
- CAMBIER J.C., NEWELL M.K., JUSTEMENT L.B., MCGUIRE J.C., LEACH K.L., CHEN Z.Z. Ia binding of ligands and cAMP stimulate nuclear translocation of PKC in B lymphocytes. Nature. 1987;327:629–632. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

