A complex web of signal-dependent trafficking underlies the triorganellar distribution of P-selectin in neuroendocrine PC12 cells
- PMID: 10385522
- PMCID: PMC2133164
- DOI: 10.1083/jcb.145.7.1419
A complex web of signal-dependent trafficking underlies the triorganellar distribution of P-selectin in neuroendocrine PC12 cells
Abstract
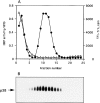
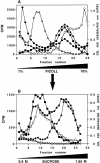
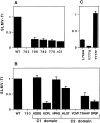
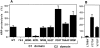
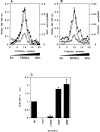
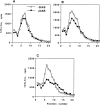
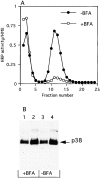
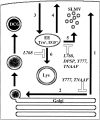
By analyzing the trafficking of HRP-P-selectin chimeras in which the lumenal domain of P-selectin was replaced with horseradish peroxidase, we determined the sequences needed for targeting to synaptic-like microvesicles (SLMV), dense core granules (DCG), and lysosomes in neuroendocrine PC12 cells. Within the cytoplasmic domain of P-selectin, Tyr777 is needed for the appearance of P-selectin in immature and mature DCG, as well as for targeting to SLMV. The latter destination also requires additional sequences (Leu768 and 786DPSP789) which are responsible for movement through endosomes en route to the SLMV. Leu768 also mediates transfer from early transferrin (Trn)-positive endosomes to the lysosomes; i.e., operates as a lysosomal targeting signal. Furthermore, SLMV targeting of HRP-P-selectin chimeras, but not the endogenous SLMV protein synaptophysin/p38, previously shown to be delivered to SLMV directly from the plasma membrane, is a Brefeldin A-sensitive process. Together, these data are consistent with a model of SLMV biogenesis which involves an endosomal intermediate in PC12 cells. In addition, we have discovered that impairment of SLMV or DCG targeting results in a concomitant increase in lysosomal delivery, illustrating the entwined relationships between routes leading to regulated secretory organelles (RSO) and to lysosomes.
Figures




References
-
- Barr FA, Huttner WB. A role for ADP-ribosylation factor 1, but not COP I, in secretory vesicles biogenesis from the trans-Golgi network. FEBS Lett. 1996;384:65–70. - PubMed
-
- Bauerfeind R, Huttner WB. Biogenesis of constitutive secretory vesicles, secretory granules and synaptic vesicles. Curr Opin Cell Biol. 1993;5:628–635. - PubMed
-
- Blagoveshchenskaya AD, Norcott JP, Cutler DF. Lysosomal targeting of P-selectin is mediated by a novel sequence within its cytoplasmic tail. J Biol Chem. 1998a;273:2729–2737. - PubMed
-
- Blagoveshchenskaya AD, Hewitt EW, Cutler DF. A balance of opposing signals within the cytoplasmic tail controls the lysosomal targeting of P-selectin. J Biol Chem. 1998b;273:27896–27903. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

