Targeting genes for self-excision in the germ line
- PMID: 10385621
- PMCID: PMC316811
- DOI: 10.1101/gad.13.12.1524
Targeting genes for self-excision in the germ line
Abstract
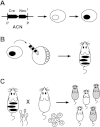
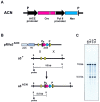
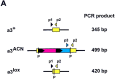
A procedure is described that directs the self-induced deletion of DNA sequences as they pass through the male germ line of mice. The testes-specific promoter from the angiotensin-converting enzyme gene was used to drive expression of the Cre-recombinase gene. Cre was linked to the selectable marker Neor, and the two genes flanked with loxP elements. This cassette was targeted to the Hoxa3 gene in mouse ES cells that were in turn used to generate chimeric mice. In these chimeras, somatic cells derived from the ES cells retained the cassette, but self-excision occurred in all ES-cell-derived sperm.
Figures


Similar articles
-
Efficient removal of loxP-flanked DNA sequences in a gene-targeted locus by transient expression of Cre recombinase in fertilized eggs.Mol Reprod Dev. 1997 Feb;46(2):109-13. doi: 10.1002/(SICI)1098-2795(199702)46:2<109::AID-MRD1>3.0.CO;2-U. Mol Reprod Dev. 1997. PMID: 9021742
-
Cre-loxP-mediated gene replacement: a mouse strain producing humanized antibodies.Curr Biol. 1994 Dec 1;4(12):1099-103. doi: 10.1016/s0960-9822(00)00248-7. Curr Biol. 1994. PMID: 7704573
-
Targeting collecting tubules using the aquaporin-2 promoter.Exp Nephrol. 1999 Jan-Feb;7(1):67-74. doi: 10.1159/000020587. Exp Nephrol. 1999. PMID: 9892817
-
Targeted viral delivery of Cre recombinase induces conditional gene deletion in cardiovascular circuits of the mouse brain.Physiol Genomics. 2004 Jun 17;18(1):25-32. doi: 10.1152/physiolgenomics.00048.2004. Epub 2004 Jun 17. Physiol Genomics. 2004. PMID: 15069166
-
Newer approaches to genetic modeling in mice: tissue-specific protein expression as studied using angiotensin-converting enzyme (ACE).Am J Pathol. 2003 Sep;163(3):807-17. doi: 10.1016/S0002-9440(10)63441-4. Am J Pathol. 2003. PMID: 12937122 Free PMC article. Review. No abstract available.
Cited by
-
Ptbp2 represses adult-specific splicing to regulate the generation of neuronal precursors in the embryonic brain.Genes Dev. 2012 Jul 15;26(14):1626-42. doi: 10.1101/gad.191338.112. Genes Dev. 2012. PMID: 22802532 Free PMC article.
-
Analysis of kinesin-2 function in photoreceptor cells using synchronous Cre-loxP knockout of Kif3a with RHO-Cre.Invest Ophthalmol Vis Sci. 2006 Nov;47(11):5039-46. doi: 10.1167/iovs.06-0032. Invest Ophthalmol Vis Sci. 2006. PMID: 17065525 Free PMC article.
-
Targeted mutagenesis of the murine transferrin receptor-2 gene produces hemochromatosis.Proc Natl Acad Sci U S A. 2002 Aug 6;99(16):10653-8. doi: 10.1073/pnas.162360699. Epub 2002 Jul 19. Proc Natl Acad Sci U S A. 2002. PMID: 12134060 Free PMC article.
-
Thyroid hormone action in the absence of thyroid hormone receptor DNA-binding in vivo.J Clin Invest. 2003 Aug;112(4):588-97. doi: 10.1172/JCI18377. J Clin Invest. 2003. PMID: 12925699 Free PMC article.
-
Characterization of Nkx6-2-derived neocortical interneuron lineages.Cereb Cortex. 2009 Jul;19 Suppl 1(Suppl 1):i1-10. doi: 10.1093/cercor/bhp038. Epub 2009 Apr 10. Cereb Cortex. 2009. PMID: 19363146 Free PMC article.
References
-
- Broach JR, Hicks JB. Replication and recombination functions associated with the yeast plasmid, 2 μ circle. Cell. 1980;21:501–508. - PubMed
-
- Capecchi MR. Human germline gene therapy: A discussion on how and why. In: Stock G, Campbell J, editors. Engineering the human germline. New York, NY: Oxford University Press; 1999. . (In press.)
-
- Colledge WH, Abella BS, Southern KW, Ratcliff R, Jiang C, Cheng SH, MacVinish LJ, Anderson JR, Cuthbert AW, Evans MJ. Generation and characterization of a ΔF508 cystic fibrosis mouse model. Nat Genet. 1995;10:445–452. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
