Interaction between FGF and BMP signaling pathways regulates development of metanephric mesenchyme
- PMID: 10385628
- PMCID: PMC316794
- DOI: 10.1101/gad.13.12.1601
Interaction between FGF and BMP signaling pathways regulates development of metanephric mesenchyme
Abstract
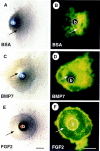
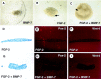
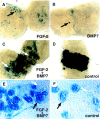
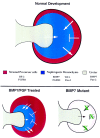
Nephrogenesis in the mouse kidney begins at embryonic day 11 and ends approximately 10 days postpartum. During this period, new nephrons are continually being generated from a stem-cell population-the nephrogenic mesenchyme-in response to signals emanating from the tips of the branching ureter. Relatively little is known about the mechanism by which the nephrogenic mesenchyme cell population is maintained at the tips of the ureter in the presence of signals promoting tubulogenesis. Previous studies have shown that a loss of Bmp7 function leads to kidney defects that are a likely result of progressive loss of nephrogenic mesenchyme by apoptosis. The studies presented here demonstrate that BMP7 signaling can prevent apoptosis in explants of metanephric mesenchyme. The surviving mesenchyme cell population, however, is not competent to respond to signals promoting tubulogenesis. In conjunction with FGF2, BMP7 promotes growth and maintains competence of the mesenchyme in vitro. In addition, FGF2 and BMP7 signaling, both independently and in combination, inhibit tubulogenesis. Interestingly, FGF2 and BMP7 also promote expansion of the stromal progenitor cell population in whole kidney culture. Because BMP7 is not produced by stromal progenitor cells, these data suggest a novel interaction between the nephrogenic mesenchyme and stromal progenitor cell populations. A model for the regulation of nephrogenesis by FGF and BMP signaling is presented.
Figures




References
-
- Barasch J, Qiao J, McWilliams G, Chen D, Oliver JA, Herzlinger D. Ureteric bud cells secrete multiple factors, including bFGF, which rescue renal progenitors from apoptosis. Am J Physiol. 1997;273:F757–767. - PubMed
-
- Cancilla B, Cauchi J, Key B, Nurcombe V, Alcorn D, Bertram J. Immunolocalization of fibroblast growth factor-1 and -2 in the embryonic rat kidney. Nephrology. 1996;2:167–174. - PubMed
-
- Coles HSR, Burne JF, Raff MC. Large-scale normal cell death in developing rat kidney and its reduction by epidermal growth factor. Development. 1993;118:969–981. - PubMed
-
- Davies JA, Garrod DR. Induction of early stages of kidney tubule differentiation by lithium ions. Dev Biol. 1995;167:50–60. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
