Method for detection and enumeration of Cryptosporidium parvum oocysts in feces, manures, and soils
- PMID: 10388670
- PMCID: PMC91423
- DOI: 10.1128/AEM.65.7.2820-2826.1999
Method for detection and enumeration of Cryptosporidium parvum oocysts in feces, manures, and soils
Abstract
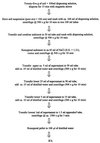
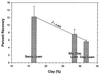
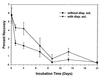
Eight concentration and purification methods were evaluated to determine percentages of recovery of Cryptosporidium parvum oocysts from calf feces. The NaCl flotation method generally resulted in the highest percentages of recovery. Based on the percentages of recovery, the amounts of fecal debris in the final oocyst preparations, the relatively short processing time (<3 h), and the low expense, the NaCl flotation method was chosen for further evaluation. Extraction efficiency was evaluated by using oocyst concentrations of 25, 50, 10(2), 10(3), 10(4), and 10(5) oocysts g of bovine feces-1. The percentages of recovery ranged from 10.8% (25 oocysts g-1) to 17.0% (10(4) oocysts g-1) (r2 = 0.996). A conservative estimate of the detection limit for bovine feces is ca. 30 oocysts g of feces-1. Percentages of recovery were determined for six different types of animal feces (cow, horse, pig, sheep, deer, and chicken feces) at a single oocyst concentration (10(4) oocysts g-1). The percentages of recovery were highest for bovine feces (17. 0%) and lowest for chicken feces (3.2%). Percentages of recovery were determined for bovine manure after 3 to 7 days of storage. The percentages of recovery ranged from 1.9 to 3.5% depending on the oocyst concentration, the time of storage, and the dispersing solution. The percentages of oocyst recovery from soils were evaluated by using different flotation solutions (NaCl, cold sucrose, ZnSO4), different dispersing solutions (Triton X-100, Tween 80, Tris plus Tween 80), different dispersion techniques (magnetic stirring, sonication, blending), and different dispersion times (5, 15, and 30 min). Twenty-five-gram soil samples were used to reduce the spatial variability. The highest percentages of recovery were obtained when we used 50 mM Tris-0.5% Tween 80 as the dispersing solution, dispersion for 15 min by stirring, and saturated NaCl as the flotation solution. The percentages of oocyst recovery from freshly spiked sandy loam, silty clay loam, and clay loam soils were ca. 12 to 18, 8, and 6%, respectively. The theoretical detection limits were ca. 1 to 2 oocysts g of soil-1 depending on the soil type. The percentages of recovery without dispersant (distilled H2O or phosphate-buffered saline) were less than 0.1%, which indicated that oocysts adhere to soil particles. The percentages of recovery decreased with storage time, although the addition of dispersant (Tris-Tween 80) before storage appeared to partially prevent adhesion. These data indicate that the NaCl flotation method is suitable for routine detection and enumeration of oocysts from feces, manures, soils, or soil-manure mixtures.
Figures
Similar articles
-
Effect of three concentration techniques on viability of Cryptosporidium parvum oocysts recovered from bovine feces.J Clin Microbiol. 1995 Oct;33(10):2592-5. doi: 10.1128/jcm.33.10.2592-2595.1995. J Clin Microbiol. 1995. PMID: 8567888 Free PMC article.
-
Recovery and enumeration of Cryptosporidium parvum from animal fecal matrices.Appl Environ Microbiol. 2003 May;69(5):2842-7. doi: 10.1128/AEM.69.5.2842-2847.2003. Appl Environ Microbiol. 2003. PMID: 12732556 Free PMC article.
-
[Isolation of Cryptosporidium parvum oocysts from fecal samples--the combination of ether extraction and discontinuous sucrose gradients].Korean J Parasitol. 1994 Mar;32(1):7-12. doi: 10.3347/kjp.1994.32.1.7. Korean J Parasitol. 1994. PMID: 8167112 Korean.
-
A rapid method for producing highly purified Cryptosporidium parvum oocysts.J Parasitol. 2007 Apr;93(2):434-6. doi: 10.1645/GE-1020R.1. J Parasitol. 2007. PMID: 17539434
-
Cryptosporidium parvum: determination of ID₅₀ and the dose-response relationship in experimentally challenged dairy calves.Vet Parasitol. 2013 Oct 18;197(1-2):104-12. doi: 10.1016/j.vetpar.2013.04.022. Epub 2013 Apr 20. Vet Parasitol. 2013. PMID: 23680540 Free PMC article.
Cited by
-
An eco-epidemiological study of contamination of soil with infective forms of intestinal parasites.Eur J Epidemiol. 2004;19(5):481-9. doi: 10.1023/b:ejep.0000027352.55755.58. Eur J Epidemiol. 2004. PMID: 15233323
-
Electrical cream separator coupled with vacuum filtration for the purification of eimerian oocysts and trichostrongylid eggs.Sci Rep. 2017 Feb 24;7:43346. doi: 10.1038/srep43346. Sci Rep. 2017. PMID: 28233853 Free PMC article.
-
Cryptosporidium spp. Are Associated with Giardia duodenalis Co-Infection in Wild and Domestic Canids.Animals (Basel). 2024 Dec 2;14(23):3484. doi: 10.3390/ani14233484. Animals (Basel). 2024. PMID: 39682449 Free PMC article.
-
Giardia duodenalis (Styles, 1902) in Cattle: Isolation of Calves with Diarrhoea and Manure Treatment in the Lagoon Presented as Risk Factors in Latvian Herds.Microorganisms. 2023 Sep 18;11(9):2338. doi: 10.3390/microorganisms11092338. Microorganisms. 2023. PMID: 37764182 Free PMC article.
-
Detection of viable Cryptosporidium parvum in soil by reverse transcription-real-time PCR targeting hsp70 mRNA.Appl Environ Microbiol. 2011 Sep;77(18):6476-85. doi: 10.1128/AEM.00677-11. Epub 2011 Jul 29. Appl Environ Microbiol. 2011. PMID: 21803904 Free PMC article.
References
-
- Arrowood M J, Sterling C R. Isolation of Cryptosporidium oocysts and sporozoites using discontinuous sucrose and isopycnic percoll gradients. J Parasitol. 1987;73:314–319. - PubMed
-
- Bloem J, Bolhuis P R, Veninga M R, Wieringa J. Microscopic methods for counting bacteria and fungi in soil. In: Kassem A, Nannipieri P, editors. Methods in applied soil microbiology and biochemistry. New York, N.Y: Academic Press; 1995. pp. 162–173.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

