Cell surface analysis techniques: What do cell preparation protocols do to cell surface properties?
- PMID: 10388679
- PMCID: PMC91432
- DOI: 10.1128/AEM.65.7.2877-2894.1999
Cell surface analysis techniques: What do cell preparation protocols do to cell surface properties?
Abstract
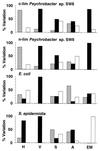
Cell surface analysis often requires manipulation of cells prior to examination. The most commonly employed procedures are centrifugation at different speeds, changes of media during washing or final resuspension, desiccation (either air drying for contact angle measurements or freeze-drying for sensitive spectroscopic analysis, such as X-ray photoelectron spectroscopy), and contact with hydrocarbon (hydrophobicity assays). The effects of these procedures on electrophoretic mobility, adhesion to solid substrata, affinity to a number of Sepharose columns, structural integrity, and cell viability were systematically investigated for a range of model organisms, including carbon- and nitrogen-limited Psychrobacter sp. strain SW8 (glycocalyx-bearing cells), Escherichia coli (gram-negative cells without a glycocalyx), and Staphylococcus epidermidis (gram-positive cells without a glycocalyx). All of the cell manipulation procedures severely modified the physicochemical properties of cells, but with each procedure some organisms were more susceptible than others. Considerable disruption of cell surfaces occurred when organisms were placed in contact with a hydrocarbon (hexadecane). The majority of cells became nonculturable after air drying and freeze-drying. Centrifugation at a high speed (15,000 x g) modified many cell surface parameters significantly, although cell viability was considerably affected only in E. coli. The type of washing or resuspension medium had a strong influence on the values of cell surface parameters, particularly when high-salt solutions were compared with low-salt buffers. The values for parameters obtained with different methods that allegedly measure similar cell surface properties did not correlate for most cells. These results demonstrate that the methods used to prepare cells for cell surface analysis need to be critically investigated for each microorganism so that the final results obtained reflect the nature of the in situ microbial cell surface as closely as possible. There is an urgent need for new, reliable, nondestructive, minimally manipulative cell surface analysis techniques that can be used in situ.
Figures









References
-
- Amory D E, Mozes N, Hermesse M P, Leonard A J, Rouxhet P G. Chemical analysis of the surface of microorganisms by X-ray photoelectron spectroscopy. FEMS Microbiol Lett. 1988;49:107–110. - PubMed
-
- Beech I B, Cheung C W S, Johnson D B, Smith J R. Comparative studies of bacterial biofilms on steel surfaces using atomic force microscopy and environmental scanning electron microscopy. Biofouling. 1996;10:65–77. - PubMed
-
- Bunt C R, Jones D S, Tucker I G. The effects of pH, ionic strength and organic phase on the bacterial adhesion to hydrocarbons (BATH) test. Int J Pharm (Amsterdam) 1993;9:93–98.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

