Physiological adaptations involved in alkane assimilation at a low temperature by Rhodococcus sp. strain Q15
- PMID: 10388690
- PMCID: PMC91443
- DOI: 10.1128/AEM.65.7.2961-2968.1999
Physiological adaptations involved in alkane assimilation at a low temperature by Rhodococcus sp. strain Q15
Abstract
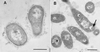
We examined physiological adaptations which allow the psychrotroph Rhodococcus sp. strain Q15 to assimilate alkanes at a low temperature (alkanes are contaminants which are generally insoluble and/or solid at low temperatures). During growth at 5 degrees C on hexadecane or diesel fuel, strain Q15 produced a cell surface-associated biosurfactant(s) and, compared to glucose-acetate-grown cells, exhibited increased cell surface hydrophobicity. A transmission electron microscopy examination of strain Q15 grown at 5 degrees C revealed the presence of intracellular electron-transparent inclusions and flocs of cells connected by an extracellular polymeric substance (EPS) when cells were grown on a hydrocarbon and morphological differences between the EPS of glucose-acetate-grown and diesel fuel-grown cells. A lectin binding analysis performed by using confocal scanning laser microscopy (CSLM) showed that the EPS contained a complex mixture of glycoconjugates, depending on both the growth temperature and the carbon source. Two glycoconjugates [beta-D-Gal-(1-3)-D-GlcNAc and alpha-L-fucose] were detected only on the surfaces of cells grown on diesel fuel at 5 degrees C. Using scanning electron microscopy, we observed strain Q15 cells on the surfaces of octacosane crystals, and using CSLM, we observed strain Q15 cells covering the surfaces of diesel fuel microdroplets; these findings indicate that this organism assimilates both solid and liquid alkane substrates at a low temperature by adhering to the alkane phase. Membrane fatty acid analysis demonstrated that strain Q15 adapted to growth at a low temperature by decreasing the degree of saturation of membrane lipid fatty acids, but it did so to a lesser extent when it was grown on hydrocarbons at 5 degrees C; these findings suggest that strain Q15 modulates membrane fluidity in response to the counteracting influences of low temperature and hydrocarbon toxicity.
Figures





References
-
- Alvarez H M, Mayer F, Fabritius D, Steinbüchel A. Formation of intracytoplasmic lipid inclusions by Rhodococcus opacus strain PD630. Arch Microbiol. 1996;165:377–386. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

