High-level production of human leptin by fed-batch cultivation of recombinant Escherichia coli and its purification
- PMID: 10388699
- PMCID: PMC91452
- DOI: 10.1128/AEM.65.7.3027-3032.1999
High-level production of human leptin by fed-batch cultivation of recombinant Escherichia coli and its purification
Abstract
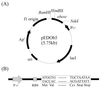
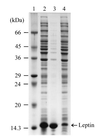
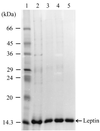
Human leptin is a 16-kDa (146-amino-acid) protein that is secreted from adipocytes and influences body weight homeostasis. In order to obtain high-level production of leptin, the human obese gene coding for leptin was expressed in Escherichia coli BL21(DE3) under the strong inducible T7 promoter. The recombinant leptin was produced as inclusion bodies in E. coli, and the recombinant leptin content was as high as 54% of the total protein content. For production of recombinant human leptin in large amounts, pH-stat fed-batch cultures were grown. Expression of leptin was induced at three different cell optical densities at 600 nm (OD600), 30, 90, and 140. When cells were induced at an OD600 of 90, the amount of leptin produced was 9.7 g/liter (37% of the total protein). After simple purification steps consisting of inclusion body isolation, denaturation and refolding, and anion-exchange chromatography, 144.9 mg of leptin that was more than 90% pure was obtained from a 50-ml culture, and the recovery yield was 41.1%.
Figures



References
-
- Altmann S W, Timans J C, Rock F L, Bazan J F, Kastelein R A. Expression and purification of a synthetic human obese gene product. Protein Express Purif. 1995;6:722–726. - PubMed
-
- Campfield L A, Smith F J, Guisez Y, Devos R, Burn P. Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neutral networks. Science. 1995;269:546–549. - PubMed
-
- Cohen S L, Halaas J L, Friedman J M, Chait B T, Bennett L, Chang D, Hecht R, Collins F. Human leptin characterization. Nature. 1996;382:589. - PubMed
-
- Curless C, Pope J, Tsai L. Effect of preinduction specific growth rate on recombinant alpha consensus interferon synthesis in Escherichia coli. Biotechnol Prog. 1990;6:149–152. - PubMed
-
- Fass R, van de Walle M, Shiloach A, Joslyn A, Kaufman J, Shiloach J. Use of high density cultures of Escherichia coli for high level production of recombinant Pseudomonas aeruginosa exotoxin A. Appl Microbiol Biotechnol. 1991;36:65–69. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

