Pharmacokinetics of dirithromycin in patients with mild or moderate cirrhosis
- PMID: 10390202
- PMCID: PMC89323
- DOI: 10.1128/AAC.43.7.1556
Pharmacokinetics of dirithromycin in patients with mild or moderate cirrhosis
Abstract
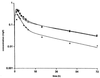
The pharmacokinetics of dirithromycin were determined over a 72-h period following oral administration of a single 500-mg dose to 8 healthy volunteers and to 16 cirrhotic patients (8 patients with class A cirrhosis and 8 patients with class B cirrhosis according to Pugh's & Child's classification). Drug levels in plasma and urine were determined by microbiological assay. The mean maximum concentrations of drug in serum obtained 3 to 4 h after administration were 0.29 +/- 0.22 mg/liter in volunteers and 0.48 +/- 0.21 and 0.52 +/- 0.38 mg/liter in patients with class A and class B cirrhosis, respectively. The elimination half-life (t1/2beta) was 23.3 +/- 7.6 h in healthy subjects and 35.2 +/- 11.8 h and 39.5 +/- 11.0 h in patients with class A and class B cirrhosis, respectively. The mean area under the concentration-time curve (AUC) and t1/2beta were significantly higher in patients with class A and B cirrhosis than in healthy controls, while total and renal clearances were markedly reduced (P < 0.01). The time to the maximum concentration of drug in serum and the volume of distribution values appeared to be similar in all groups, and the mean recovery in urine at 72 h ranged from 3.7 to 5.7%, without significant differences among groups. These results demonstrate that some dirithromycin kinetic parameters are significantly different in cirrhotic patients in comparison to those in healthy volunteers. However, an increase in the t1/2beta or AUC, which is also observed with other semisynthetic macrolides (e.g., azithromycin), does seem to be not clinically relevant if one takes into account both the high therapeutic indices of these antibiotics and the usually short duration of therapy. Therefore, on the limited basis of single-dose administration, no modifications of dirithromycin dosage seem to be required even for patients with class B liver cirrhosis.
Figures
References
-
- Brogden R N, Peters D H. Dirithromycin: a review of its antimicrobial activity, pharmacokinetic properties and therapeutic efficacy. Drugs. 1994;48:599–616. - PubMed
-
- Chu S Y, Granneman G R, Pichotta P J, Decourt J P, Girault J, Fourtillan J B. Effect of moderate or severe hepatic impairment on clarithromycin pharmacokinetics. J Clin Pharmacol. 1993;33:480–485. - PubMed
-
- Counter F T, Ensminger P W, Preston D A, Wu C-Y E, Greene J M, Felty-Duckworth A M, Paschal J W, Kirst H A. Synthesis and antimicrobial evaluation of dirithromycin (AS-E 136; LY237216), a new macrolide antibiotic derived from erythromycin. Antimicrob Agents Chemother. 1991;35:1116–1126. - PMC - PubMed
-
- Gibaldi M, Perrier D. Pharmacokinetics. 2nd ed. New York, N.Y: Marcel Dekker, Inc.; 1982.
-
- Kucers A, Bennett N. The use of antibiotics. A comprehensive review with clinical emphasis. 4th ed. London, United Kingdom: William Heinemann; 1987. Erythromycin; pp. 851–882.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical