Efficacy of locally delivered polyclonal immunoglobulin against Pseudomonas aeruginosa peritonitis in a murine model
- PMID: 10390211
- PMCID: PMC89332
- DOI: 10.1128/AAC.43.7.1609
Efficacy of locally delivered polyclonal immunoglobulin against Pseudomonas aeruginosa peritonitis in a murine model
Abstract
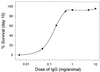
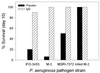
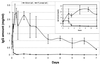
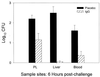
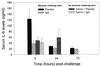
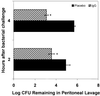
Infectious peritonitis results from bacterial contamination of the abdominal cavity. Conventional antibiotic treatment is complicated both by the emergence of antibiotic-resistant bacteria and by increased patient populations intrinsically at risk for nosocomial infections. To complement antibiotic therapies, the efficacy of direct, locally applied pooled human immunoglobulin G (IgG) was assessed in a murine model (strains CF-1, CD-1, and CFW) of peritonitis caused by intraperitoneal inoculations of 10(6) or 10(7) CFU of Pseudomonas aeruginosa (strains IFO-3455, M-2, and MSRI-7072). Various doses of IgG (0.005 to 10 mg/mouse) administered intraperitoneally simultaneously with local bacterial challenge significantly increased survival in a dose-dependent manner. Local intraperitoneal application of 10 mg of IgG increased animal survival independent of either the P. aeruginosa or the murine strains used. A local dose of 10 mg of IgG administered up to 6 h prophylactically or at the time of bacterial challenge resulted in 100% survival. Therapeutic 10-mg IgG treatment given up to 12 h postinfection also significantly increased survival. Human IgG administered to the mouse peritoneal cavity was rapidly detected systemically in serum. Additionally, administered IgG in peritoneal lavage fluid samples actively opsonized and decreased the bacterial burden via phagocytosis at 2 and 4 h post-bacterial challenge. Tissue microbial quantification studies showed that 1.0 mg of locally applied IgG significantly reduced the bacterial burden in the liver, peritoneal cavity, and blood and correlated with reduced levels of interleukin-6 in serum.
Figures


Similar articles
-
Efficacy of locally delivered polyclonal immunoglobulin against Pseudomonas aeruginosa infection in a murine burn wound model.Burns. 1999 Aug;25(5):415-23. doi: 10.1016/s0305-4179(99)00017-0. Burns. 1999. PMID: 10439150
-
Prophylactic and therapeutic efficacy of immunoglobulin G antibodies to Pseudomonas aeruginosa lipopolysaccharide against murine experimental corneal infection.Invest Ophthalmol Vis Sci. 1997 Jun;38(7):1418-25. Invest Ophthalmol Vis Sci. 1997. PMID: 9191605
-
Prophylactic treatment of gram-positive and gram-negative abdominal implant infections using locally delivered polyclonal antibodies.J Biomed Mater Res. 2002 Apr;60(1):206-15. doi: 10.1002/jbm.10069. J Biomed Mater Res. 2002. PMID: 11835177
-
Pharmacodynamic and protective properties of a murine lipopolysaccharide-specific monoclonal antibody in experimental Pseudomonas aeruginosa pneumonia in mice.Microbiol Immunol. 1991;35(12):1131-41. doi: 10.1111/j.1348-0421.1991.tb01634.x. Microbiol Immunol. 1991. PMID: 1808464
-
Pseudomonas aeruginosa chromosomal beta-lactamase in patients with cystic fibrosis and chronic lung infection. Mechanism of antibiotic resistance and target of the humoral immune response.APMIS Suppl. 2003;(116):1-47. APMIS Suppl. 2003. PMID: 14692154 Review.
Cited by
-
Experimental manipulation of transforming growth factor-beta isoforms significantly affects adhesion formation in a murine surgical model.Am J Pathol. 2005 Oct;167(4):1005-19. doi: 10.1016/s0002-9440(10)61190-x. Am J Pathol. 2005. PMID: 16192636 Free PMC article.
-
Polyvalent human immunoglobulin for infectious diseases: Potential to circumvent antimicrobial resistance.Front Immunol. 2023 Jan 9;13:987231. doi: 10.3389/fimmu.2022.987231. eCollection 2022. Front Immunol. 2023. PMID: 36713426 Free PMC article. Review.
-
Antibacterial efficacy of R-type pyocins towards Pseudomonas aeruginosa in a murine peritonitis model.Antimicrob Agents Chemother. 2008 May;52(5):1647-52. doi: 10.1128/AAC.01479-07. Epub 2008 Mar 10. Antimicrob Agents Chemother. 2008. PMID: 18332164 Free PMC article.
-
A Single B-repeat of Staphylococcus epidermidis accumulation-associated protein induces protective immune responses in an experimental biomaterial-associated infection mouse model.Clin Vaccine Immunol. 2014 Sep;21(9):1206-14. doi: 10.1128/CVI.00306-14. Epub 2014 Jun 11. Clin Vaccine Immunol. 2014. PMID: 24920603 Free PMC article.
-
Requirement of the Pseudomonas aeruginosa CbrA sensor kinase for full virulence in a murine acute lung infection model.Infect Immun. 2014 Mar;82(3):1256-67. doi: 10.1128/IAI.01527-13. Epub 2013 Dec 30. Infect Immun. 2014. PMID: 24379284 Free PMC article.
References
-
- Barriere S L, Guglielmo B J. Gram-negative sepsis, the sepsis syndrome, and the role of antiendotoxin monoclonal antibodies. Clin Pharmacol. 1992;11:223–235. - PubMed
-
- Beutler B, Milsark I W, Cerami A C. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science. 1985;229:869–871. - PubMed
-
- Bodey G P, Jadeja L, Elting L. Pseudomonas bacteremia. Retrospective analysis of 410 episodes. Arch Intern Med. 1985;145:1621–1629. - PubMed
-
- Calandra T, Gerain J, Heumann D, Baumgartner J-D, Glauser M P. High circulating levels of interleukin-6 in patients with septic shock: evolution during sepsis, prognostic value, and interplay with other cytokines. Am J Med. 1991;91:23–29. - PubMed
-
- Clapp D W, Kliegman R M, Baley J E, Shenker N, Kyllonen K, Fanaroff A A, Berger M. Use of intravenously administered immune globulin to prevent nosocomial sepsis in low birth weight infants: report of a pilot study. J Pediatr. 1989;115:973–978. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

