Single-dose pharmacokinetics and safety of abacavir (1592U89), zidovudine, and lamivudine administered alone and in combination in adults with human immunodeficiency virus infection
- PMID: 10390227
- PMCID: PMC89348
- DOI: 10.1128/AAC.43.7.1708
Single-dose pharmacokinetics and safety of abacavir (1592U89), zidovudine, and lamivudine administered alone and in combination in adults with human immunodeficiency virus infection
Abstract
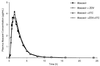
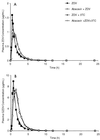
Abacavir (1592U89), a nucleoside reverse transcriptase inhibitor with in vitro activity against human immunodeficiency virus type-1 (HIV-1), has been evaluated for efficacy and safety in combination regimens with other nucleoside analogs, including zidovudine (ZDV) and lamivudine (3TC). To evaluate the potential pharmacokinetic interactions between these agents, 15 HIV-1-infected adults with a median CD4(+) cell count of 347 cells/mm3 (range, 238 to 570 cells/mm3) were enrolled in a randomized, seven-period crossover study. The pharmacokinetics and safety of single doses of abacavir (600 mg), ZDV (300 mg), and 3TC (150 mg) were evaluated when each drug was given alone or when any two or three drugs were given concurrently. The concentrations of all drugs in plasma and the concentrations of ZDV and its 5'-glucuronide metabolite, GZDV, in urine were measured for up to 24 h postdosing, and pharmacokinetic parameter values were calculated by noncompartmental methods. The maximum drug concentration (Cmax), the area under the concentration-time curve from time zero to infinity (AUC0-infinity), time to Cmax (Tmax), and apparent elimination half-life (t1/2) of abacavir in plasma were unaffected by coadministration with ZDV and/or 3TC. Coadministration of abacavir with ZDV (with or without 3TC) decreased the mean Cmax of ZDV by approximately 20% (from 1.5 to 1.2 microg/ml), delayed the median Tmax for ZDV by 0.5 h, increased the mean AUC0-infinity for GZDV by up to 40% (from 11.8 to 16.5 microg. h/ml), and delayed the median Tmax for GZDV by approximately 0.5 h. Coadministration of abacavir with 3TC (with or without ZDV) decreased the mean AUC0-infinity for 3TC by approximately 15% (from 5.1 to 4.3 microg. h/ml), decreased the mean Cmax by approximately 35% (from 1.4 to 0.9 microg/ml), and delayed the median Tmax by approximately 1 h. While these changes were statistically significant, they are similar to the effect of food intake (for ZDV) or affect an inactive metabolite (for GZDV) or are relatively minor (for 3TC) and are therefore not considered to be clinically significant. No significant differences were found in the urinary recoveries of ZDV or GZDV when ZDV was coadministered with abacavir. There was no pharmacokinetic interaction between ZDV and 3TC. Mild to moderate headache, nausea, lymphadenopathy, hematuria, musculoskeletal chest pain, neck stiffness, and fever were the most common adverse events reported by those who received abacavir. Coadministration of ZDV or 3TC with abacavir did not alter this adverse event profile. The three-drug regimen was primarily associated with gastrointestinal events. In conclusion, no clinically significant pharmacokinetic interactions occurred between abacavir, ZDV, and 3TC in HIV-1-infected adults. Coadministration of abacavir with ZDV or 3TC produced mild changes in the absorption and possibly the urinary excretion characteristics of ZDV-GZDV and 3TC that were not considered to be clinically significant. Coadministration of abacavir with ZDV and/or 3TC was generally well tolerated and did not produce unexpected adverse events.
Figures

Similar articles
-
A comparison of the steady-state pharmacokinetics and safety of abacavir, lamivudine, and zidovudine taken as a triple combination tablet and as abacavir plus a lamivudine-zidovudine double combination tablet by HIV-1-infected adults.Pharmacotherapy. 2001 Apr;21(4):424-30. doi: 10.1592/phco.21.5.424.34497. Pharmacotherapy. 2001. PMID: 11310515 Clinical Trial.
-
Abacavir/lamivudine/zidovudine as a combined formulation tablet: bioequivalence compared with each component administered concurrently and the effect of food on absorption.J Clin Pharmacol. 2001 Mar;41(3):277-88. doi: 10.1177/00912700122010096. J Clin Pharmacol. 2001. PMID: 11269568 Clinical Trial.
-
A randomized, double-blind study of triple nucleoside therapy of abacavir, lamivudine, and zidovudine versus lamivudine and zidovudine in previously treated human immunodeficiency virus type 1-infected children. The CNAA3006 Study Team.Pediatrics. 2001 Jan;107(1):E4. doi: 10.1542/peds.107.1.e4. Pediatrics. 2001. PMID: 11134468 Clinical Trial.
-
Zidovudine/Lamivudine vs. Abacavir/Lamivudine vs. Tenofovir/Emtricitabine in fixed-dose combinations as initial treatment for HIV patients: a systematic review and network meta-analysis.Colomb Med (Cali). 2017 Jun 30;48(2):70-81. Colomb Med (Cali). 2017. PMID: 29021641 Free PMC article.
-
A review of the pharmacokinetics of abacavir.Clin Pharmacokinet. 2008;47(6):351-71. doi: 10.2165/00003088-200847060-00001. Clin Pharmacokinet. 2008. PMID: 18479171 Review.
Cited by
-
HLA-B*57:01 screening and hypersensitivity reaction to abacavir between 1999 and 2016 in the OPERA® observational database: a cohort study.AIDS Res Ther. 2019 Jan 16;16(1):1. doi: 10.1186/s12981-019-0217-3. AIDS Res Ther. 2019. PMID: 30651100 Free PMC article.
-
Domestic cat model for predicting human nucleoside analogue pharmacokinetics in blood and seminal plasma.Antimicrob Agents Chemother. 2001 Jul;45(7):2173-6. doi: 10.1128/AAC.45.7.2173-2176.2001. Antimicrob Agents Chemother. 2001. PMID: 11408248 Free PMC article.
-
Development and characterization of a long-acting nanoformulated abacavir prodrug.Nanomedicine (Lond). 2016 Aug;11(15):1913-27. doi: 10.2217/nnm-2016-0164. Epub 2016 Jul 26. Nanomedicine (Lond). 2016. PMID: 27456759 Free PMC article.
-
Long-term exposure to zidovudine delays cell cycle progression, induces apoptosis, and decreases telomerase activity in human hepatocytes.Toxicol Sci. 2009 Sep;111(1):120-30. doi: 10.1093/toxsci/kfp136. Epub 2009 Jun 18. Toxicol Sci. 2009. PMID: 19541796 Free PMC article.
-
Tolerabilities of antiretrovirals in paediatric HIV infection.Drug Saf. 2002;25(14):973-91. doi: 10.2165/00002018-200225140-00001. Drug Saf. 2002. PMID: 12408730 Review.
References
-
- Angel J B, Hussey E K, Hall S T, Donn K H, Morris D M, McCormack J P, Montaner J S G, Ruedy J. Pharmacokinetics of 3TC (GR 109714X) administered with and without food to HIV-infected patients. Drug Invest. 1993;6:70–74.
-
- Blum M R, Liao S H T, Good S S, De Miranda P. Pharmacokinetics and bioavailability of zidovudine in humans. Am J Med. 1988;85(Suppl. 2A):189–194. - PubMed
-
- Carpenter C C, Fischl M A, Hammer S M, Hirsch M S, Jacobsen D M, Katzenstein D A, Montaner J S, Richman D D, Saag M S, Schooley R T, Thompson M A, Vella S, Yeni P G, Volberding P A. Antiretroviral therapy for HIV infection in 1997. Updated recommendations of the International AIDS Society-USA Panel. JAMA. 1997;277:1962–1969. - PubMed
-
- Collins J M, Unadkat J D. Clinical pharmacokinetics of zidovudine. An overview of current data. Clin Pharmacokinet. 1989;17:1–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

