Significance of fatty acids in pregnancy-induced immunosuppression
- PMID: 10391868
- PMCID: PMC95733
- DOI: 10.1128/CDLI.6.4.587-593.1999
Significance of fatty acids in pregnancy-induced immunosuppression
Abstract
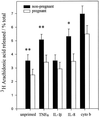
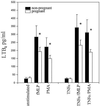
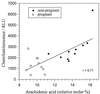
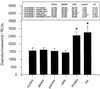
Pregnancy can exert suppressive effects on chronic inflammatory conditions. We have previously demonstrated a depression in polymorphonuclear leukocyte (PMN) respiratory burst during pregnancy which could explain this amelioration. To elucidate the biochemical mechanism, we have examined PMN phospholipase A2 (PLA2) activity and its relationship to cellular and circulating fatty acids in pregnant women (30 to 34 weeks) and nonpregnant controls. PMN PLA2 activity was determined by arachidonic acid (AA) and leukotriene B4 (LTB4) release, respiratory burst activity was determined by lucigenin-enhanced chemiluminescence, and total serum and PMN fatty acid levels were determined by gas-liquid chromatography. AA release was significantly reduced for pregnancy PMNs in response to N-formyl-met-leu-phe (fMLP) under unprimed and tumor necrosis factor alpha (TNF-alpha)- or interleukin 8-primed conditions. Similarly, LTB4 liberation was significantly reduced in response to fMLP and phorbol myristate acetate in unprimed and TNF-alpha-primed pregnancy PMNs. All major fatty acid classes were altered in the pregnant state. Of these differences in PMNs, oleic acid and alpha-linolenic acid showed a significant increase (13 and 26%, respectively) and stearic acid and AA showed a significant decrease (8 and 30%, respectively). The stearic acid, oleic acid, and AA compositions of all cells analyzed correlated with their corresponding changes in serum fatty acid levels. Crossover serum incubations modified both fatty acid profiles and the PMN respiratory burst accordingly, while individual fatty acid incorporation studies highlighted the importance of polyunsaturated fatty acids for NADPH oxidase efficiency. These findings indicate that the attenuation of PMN function in pregnancy may originate from a reduction in the available pool of cellular fatty acids. Furthermore, this reduction arises as a direct result of a pregnancy-induced shift in circulating fatty acids from polyunsaturated to monounsaturated forms.
Figures



References
-
- Andresen R H, Monroe G W. Experimental study of the behavior of adult human skin homografts during pregnancy. Am J Obstet Gynecol. 1962;84:1096–1103. - PubMed
-
- Barnette M S, Rush J, Marshall L A, Foley J J, Schmidt D B, Sarau H M. Effects of Scalaradial, a novel inhibitor of 14KDa phospholipase A2 on human neutrophil function. Biochem Pharmacol. 1994;47:1661–1668. - PubMed
-
- Beilin L J, Croft K D, Michael C A, Ritchie J, Schmidt L, Vandongen R, Walter B N J. Neutrophil platelet activating factor in normal and hypertensive pregnancy and in pregnancy-induced hypertension. Clin Sci. 1993;85:63–70. - PubMed
-
- Bellavite P, Guarini P, Biasi D, Carletto A, Trevisan M T, Caramaschi P, Bambara L M, Corrocher R. Correlations between the intensity of fMLP-dependent respiratory burst and cellular fatty acid composition in human neutrophils. Br J Haematol. 1995;89:271–276. - PubMed
-
- Bjorksten B, Soderstrom T, Damber M-G, Von Schoultz B, Stigbrand T. Polymorphonuclear leucocyte function during pregnancy. Scand J Immunol. 1978;8:257–262. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

