Active participation of CCR5(+)CD8(+) T lymphocytes in the pathogenesis of liver injury in graft-versus-host disease
- PMID: 10393698
- PMCID: PMC408408
- DOI: 10.1172/JCI6642
Active participation of CCR5(+)CD8(+) T lymphocytes in the pathogenesis of liver injury in graft-versus-host disease
Abstract
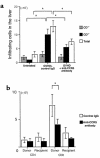
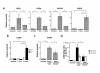
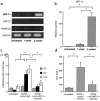
We examined the molecular pathogenesis of graft-versus-host disease-associated (GVHD-associated) liver injury in mice, focusing on the role of chemokines. At the second week after cell transfer in the parent-into-F1 model of GVHD, CD8(+) T cells -- especially donor-derived CD8(+) T cells -- infiltrated the liver, causing both portal hepatitis and nonsuppurative destructive cholangitis (NSDC). These migrating cells expressed CCR5. Moreover, macrophage inflammatory protein-1alpha (MIP-1alpha), one of the ligands for CCR5, was selectively expressed on intralobular bile duct epithelial cells, endothelial cells, and infiltrating macrophages and lymphocytes. Administration of anti-CCR5 antibody dramatically reduced the infiltration of CCR5(+)CD8(+) T lymphocytes into the liver, and consequently protected against liver damage in GVHD. The levels of Fas ligand (FasL) mRNA expression in the liver were also decreased by anti-CCR5 antibody treatment. Anti-MIP-1alpha antibody treatment also reduced liver injury. These results suggest that MIP-1alpha-induced migration of CCR5-expressing CD8(+) T cells into the portal areas of the liver plays a significant role in causing liver injury in GVHD; thus, CCR5 and its ligand may be the novel target molecules of therapeutic intervention of hepatic GVHD.
Figures


References
-
- Ferrara JLM, Deeg HJ. Graft-versus-host disease. N Engl J Med. 1991;324:667–674. - PubMed
-
- Gleichmann E, Pals ST, Rolink AG, Radaszkiewicz T, Gleichmann H. Graft-versus-host reactions: clues to the ethiopathology of a spectrum of immunological diseases. Immunol Today. 1984;5:324–332. - PubMed
-
- Rus V, Svetic A, Nguyen P, Gause WC, Via CS. Kinetics of Th1 and Th2 cytokine production during the early course of acute and chronic murine graft-versus-host disease. J Immunol. 1995;155:2396–2406. - PubMed
-
- Bobe P, et al. Fas-mediated liver damage in MRL hemopoietic chimeras undergoing lpr-mediated graft-versus-host disease. J Immunol. 1997;159:4197–4204. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

