Nitric oxide inhibits HIV tat-induced NF-kappaB activation
- PMID: 10393859
- PMCID: PMC1866645
- DOI: 10.1016/s0002-9440(10)65121-8
Nitric oxide inhibits HIV tat-induced NF-kappaB activation
Abstract
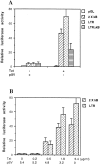
To evaluate the roles of nitric oxide (NO) on human immunodeficiency virus (HIV) Tat-induced transactivation of HIV long terminal repeat (HIV-LTR), we examined the effect of NO in the regulation of nuclear factor (NF)-kappaB, a key transcription factor involved in HIV gene expression and viral replication. In the present study, we demonstrate that HIV Tat activates NF-kappaB and that this activation can be attenuated by endogenous or exogenous NO. Inhibition of endogenous NO production with the NO synthase (NOS) inhibitor L-NMMA causes a significant increase in Tat-induced NF-kappaB activity. In addition, NO attenuates signal-initiated degradation of IkappaBalpha, an intracellular inhibitor of NF-kappaB, and blocks the DNA binding activity of the NF-kappaB p50/p50 homodimer and p50/p65 heterodimer. To determine how NO is induced by HIV Tat, reverse transcription polymerase chain reaction was used to demonstrate the induction of NOS-2 and NOS-3 mRNA by Tat. Although a putative NF-kappaB binding site was identified in the -74 GGAGAGCCCCC -64 region of the NOS-3 gene promoter, gel mobility shift assays and site-directed mutation analyses suggest that the putative NF-kappaB site is not of primary importance. Rather, several Sp-1 sites adjoining the putative NF-kappaB binding site in the promoter region of NOS-3 gene are required for the induction of NOS-3 gene expression by Tat.
Figures









References
-
- MacMicking J, Xie QW, Nathan C: Nitric oxide and macrophage function. Annu Rev Immunol 1997, 15:323-350 - PubMed
-
- Guidotti LG, Chisari FV: To kill or to cure: options in host defense against viral infection. Curr Opin Immunol 1996, 8:478-483 - PubMed
-
- Peterhans E: Reactive oxygen species and nitric oxide in viral diseases. Biol Trace Element Res 1997, 56:107-116 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

