Supraspinal contributions to hyperalgesia
- PMID: 10393881
- PMCID: PMC33602
- DOI: 10.1073/pnas.96.14.7687
Supraspinal contributions to hyperalgesia
Abstract
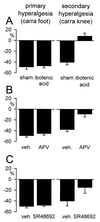
Tissue injury is associated with sensitization of nociceptors and subsequent changes in the excitability of central (spinal) neurons, termed central sensitization. Nociceptor sensitization and central sensitization are considered to underlie, respectively, development of primary hyperalgesia and secondary hyperalgesia. Because central sensitization is considered to reflect plasticity at spinal synapses, the spinal cord has been the principal focus of studies of mechanisms of hyperalgesia. Not surprisingly, glutamate, acting at a spinal N-methyl-D-aspartate (NMDA) receptor, has been implicated in development of secondary hyperalgesia associated with somatic, neural, and visceral structures. Downstream of NMDA receptor activation, spinal nitric oxide (NO.), protein kinase C, and other mediators have been implicated in maintaining such hyperalgesia. Accumulating evidence, however, reveals a significant contribution of supraspinal influences to development and maintenance of hyperalgesia. Spinal cord transection prevents development of secondary, but not primary, mechanical and/or thermal hyperalgesia after topical mustard oil application, carrageenan inflammation, or nerve-root ligation. Similarly, inactivation of the rostral ventromedial medulla (RVM) attenuates hyperalgesia and central sensitization in several models of persistent pain. Inhibition of medullary NMDA receptors or NO. generation attenuates somatic and visceral hyperalgesia. In support, topical mustard oil application or colonic inflammation increases expression of NO. synthase in the RVM. These data suggest a prominent role for the RVM in mediating the sensitization of spinal neurons and development of secondary hyperalgesia. Results to date suggest that peripheral injury and persistent input engage spinobulbospinal mechanisms that may be the prepotent contributors to central sensitization and development of secondary hyperalgesia.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

