A more unified picture for the thermodynamics of nucleic acid duplex melting: a characterization by calorimetric and volumetric techniques
- PMID: 10393911
- PMCID: PMC22151
- DOI: 10.1073/pnas.96.14.7853
A more unified picture for the thermodynamics of nucleic acid duplex melting: a characterization by calorimetric and volumetric techniques
Abstract
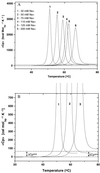
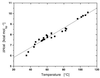
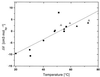
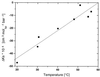
We use a combination of calorimetric and volumetric techniques to detect and to characterize the thermodynamic changes that accompany helix-to-coil transitions for five polymeric nucleic acid duplexes. Our calorimetric measurements reveal that melting of the duplexes is accompanied by positive changes in heat capacity (DeltaCP) of similar magnitude, with an average DeltaCP value of 64.6 +/- 21.4 cal deg-1 mol-1. When this heat capacity value is used to compare significantly different transition enthalpies (DeltaHo) at a common reference temperature, Tref, we find DeltaHTref for duplex melting to be far less dependent on duplex type, base composition, or base sequence than previously believed on the basis of the conventional assumption of a near-zero value for DeltaCP. Similarly, our densimetric and acoustic measurements reveal that, at a given temperature, all the AT- and AU-containing duplexes studied here melt with nearly the same volume and compressibility changes. In the aggregate, our results, in conjunction with literature data, suggest a more unified picture for the thermodynamics of nucleic acid duplex melting. Specifically, when compared at a common temperature, the apparent large differences present in the literature for the transition enthalpies of different duplexes become much more compressed, and the melting of all-AT- and all-AU-containing duplexes exhibits similar volume and compressibility changes despite differences in sequence and conformation. Thus, insofar as thermodynamic properties are concerned, when comparing duplexes, the temperature under consideration is as important as, if not more important than, the duplex type, the base composition, or the base sequence. This general behavior has significant implications for our basic understanding of the forces that stabilize nucleic acid duplexes. This behavior also is of practical significance in connection with the use of thermodynamic databases for designing probes and for assessing the affinity and specificity associated with hybridization-based protocols used in a wide range of sequencing, diagnostic, and therapeutic applications.
Figures
References
-
- Breslauer K J. In: Thermodynamic Data for Biochemistry and Biotechnology. Hinz H-J, editor. Berlin: Springer; 1986. pp. 402–427.
-
- Filimonov V V. In: Thermodynamic Data for Biochemistry and Biotechnology. Hinz H-J, editor. Berlin: Springer; 1986. pp. 377–401.
-
- Klump H H. In: Biochemical Thermodynamics. Jones M N, editor. Amsterdam: Elsevier; 1988. pp. 100–144.
-
- Klump H H, Völker J, Maeder D L, Niermann Th, Sobolewski C H M. Thermochim Acta. 1991;193:391–415.
-
- Plum G E, Breslauer K J. Curr Opin Struct Biol. 1995;5:682–690. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

