Five-transmembrane domains appear sufficient for a G protein-coupled receptor: functional five-transmembrane domain chemokine receptors
- PMID: 10393923
- PMCID: PMC22163
- DOI: 10.1073/pnas.96.14.7922
Five-transmembrane domains appear sufficient for a G protein-coupled receptor: functional five-transmembrane domain chemokine receptors
Abstract
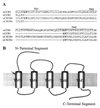
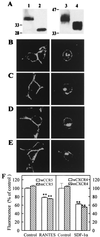
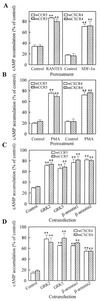
The putative seven-transmembrane (TM) domains have been the structural hallmark for the superfamily of heterotrimeric G protein-coupled receptors (GPCRs) that regulate a variety of cellular functions by mediating a large number of extracellular signals. Five-TM GPCR mutants of chemokine receptor CCR5 and CXCR4, the N-terminal segment of which connected directly to TM3 as a result of a deletion of TM1-2 and the first intracellular and extracellular loops, have been obtained in this study. Laser confocal microscopy and flow cytometry analysis revealed that these five-TM mutant GPCRs were expressed stably on the cell surface after transfection into human embryonic kidney 293 cells. The five-TM CCR5 and CXCR4 functioned as normal chemokine receptors in mediating chemokine-stimulated chemotaxis, Ca2+ influx, and activation of pertussis toxin-sensitive G proteins. Like the wild-type GPCRs, the five-TM mutant receptors also underwent agonist-dependent internalization and desensitization and were subjected to regulation by GPCR kinases and arrestins. Our study indicates that five-TM domains, at least in the case of CCR5 and CXCR4, appear to meet the minimum structural requirements for a functional GPCR and suggests possible existence of functional five-TM GPCRs in nature during evolution.
Figures


References
-
- Bargmann C I. Science. 1998;282:2028–2033. - PubMed
-
- Wess J. FASEB J. 1997;11:346–354. - PubMed
-
- Ji T H, Grossmann M, Ji I. J Biol Chem. 1998;273:17299–17302. - PubMed
-
- Baggiolini M. Nature (London) 1998;392:565–568. - PubMed
-
- Moser B, Loetscher M, Piali L, Loetscher P. Int Rev Immunol. 1998;16:323–344. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

