Nephrin is specifically located at the slit diaphragm of glomerular podocytes
- PMID: 10393930
- PMCID: PMC22170
- DOI: 10.1073/pnas.96.14.7962
Nephrin is specifically located at the slit diaphragm of glomerular podocytes
Abstract
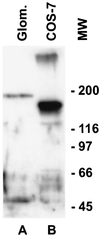
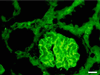
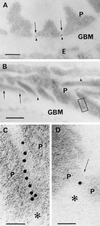
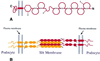
We describe here the size and location of nephrin, the first protein to be identified at the glomerular podocyte slit diaphragm. In Western blots, nephrin antibodies generated against the two terminal extracellular Ig domains of recombinant human nephrin recognized a 180-kDa protein in lysates of human glomeruli and a 150-kDa protein in transfected COS-7 cell lysates. In immunofluorescence, antibodies to this transmembrane protein revealed reactivity in the glomerular basement membrane region, whereas the podocyte cell bodies remained negative. In immunogold-stained thin sections, nephrin label was found at the slit between podocyte foot processes. The congenital nephrotic syndrome of the Finnish type (NPHS1), a disease in which the nephrin gene is mutated, is characterized by massive proteinuria already in utero and lack of slit diaphragm and foot processes. These features, together with the now demonstrated localization of nephrin to the slit diaphragm area, suggests an essential role for this protein in the normal glomerular filtration barrier. A zipper-like model for nephrin assembly in the slit diaphragm is discussed, based on the present and previous data.
Figures

References
-
- Tisher C C, Madsen K M. In: Anatomy of the Kidney. Brenner B M, Rector F C, editors. Vol. 1. Philadelphia: Saunders; 1996. pp. 3–71.
-
- Brenner B M, Hostetter T H, Humes H D. N Engl J Med. 1978;298:826–832. - PubMed
-
- Batsford S R, Rohrbach R, Vogt A. Kidney Int. 1987;31:710–717. - PubMed
-
- Kanwar Y S, Liu Z Z, Wallner E I. Semin Nephrol. 1991;11:390–413. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

