Synaptic depression creates a switch that controls the frequency of an oscillatory circuit
- PMID: 10393973
- PMCID: PMC22213
- DOI: 10.1073/pnas.96.14.8206
Synaptic depression creates a switch that controls the frequency of an oscillatory circuit
Abstract
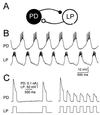
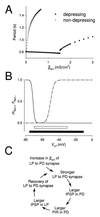
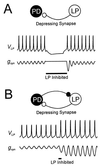
Synaptic depression is a form of short-term plasticity exhibited by many synapses. Nonetheless, the functional significance of synaptic depression in oscillatory networks is not well understood. We show that, in a recurrent inhibitory network that includes an intrinsic oscillator, synaptic depression can give rise to two distinct modes of network operation. When the maximal conductance of the depressing synapse is small, the oscillation period is determined by the oscillator component. Increasing the maximal conductance beyond a threshold value activates a positive-feedback mechanism that greatly enhances the synaptic strength. In this mode, the oscillation period is determined by the strength and dynamics of the depressing synapse. Because of the regenerative nature of the feedback mechanism, the circuit can be switched from one mode of operation to another by a very small change in the maximal conductance of the depressing synapse. Our model was inspired by experimental work on the pyloric network of the lobster. The pyloric network produces a simple motor rhythm generated by a pacemaker neuron that receives feedback inhibition from a depressing synapse. In some preparations, elimination of the synapse had no effect on the period of the rhythm, whereas in other preparations, there was a significant decrease in the period. We propose that the pyloric network can operate in either of the two modes suggested by the model, depending on the maximal conductance of the depressing synapse.
Figures


Similar articles
-
Mechanisms of oscillation in dynamic clamp constructed two-cell half-center circuits.J Neurophysiol. 1996 Aug;76(2):867-83. doi: 10.1152/jn.1996.76.2.867. J Neurophysiol. 1996. PMID: 8871205
-
Activity-dependent modification of inhibitory synapses in models of rhythmic neural networks.Nat Neurosci. 2001 Mar;4(3):297-303. doi: 10.1038/85147. Nat Neurosci. 2001. PMID: 11224547
-
Synaptic depression mediates bistability in neuronal networks with recurrent inhibitory connectivity.J Neurosci. 2001 Dec 1;21(23):9460-70. doi: 10.1523/JNEUROSCI.21-23-09460.2001. J Neurosci. 2001. PMID: 11717380 Free PMC article.
-
Maturation of rhythmic neural network: role of central modulatory inputs.J Physiol Paris. 2003 Jan;97(1):59-68. doi: 10.1016/j.jphysparis.2003.10.007. J Physiol Paris. 2003. PMID: 14706691 Review.
-
Oscillations and oscillatory behavior in small neural circuits.Biol Cybern. 2006 Dec;95(6):537-54. doi: 10.1007/s00422-006-0125-1. Epub 2006 Dec 7. Biol Cybern. 2006. PMID: 17151878 Review.
Cited by
-
Functional consequences of animal-to-animal variation in circuit parameters.Nat Neurosci. 2009 Nov;12(11):1424-30. doi: 10.1038/nn.2404. Epub 2009 Oct 18. Nat Neurosci. 2009. PMID: 19838180 Free PMC article.
-
Neuromodulator-evoked synaptic metaplasticity within a central pattern generator network.J Neurophysiol. 2012 Nov;108(10):2846-56. doi: 10.1152/jn.00586.2012. Epub 2012 Aug 29. J Neurophysiol. 2012. PMID: 22933725 Free PMC article.
-
Short-Term Synaptic Plasticity at Interneuronal Synapses Could Sculpt Rhythmic Motor Patterns.Front Neural Circuits. 2016 Feb 3;10:4. doi: 10.3389/fncir.2016.00004. eCollection 2016. Front Neural Circuits. 2016. PMID: 26869889 Free PMC article.
-
Short-term synaptic plasticity compensates for variability in number of motor neurons at a neuromuscular junction.J Neurosci. 2012 Nov 7;32(45):16007-17. doi: 10.1523/JNEUROSCI.2584-12.2012. J Neurosci. 2012. PMID: 23136437 Free PMC article.
-
Xolotl: An Intuitive and Approachable Neuron and Network Simulator for Research and Teaching.Front Neuroinform. 2018 Nov 26;12:87. doi: 10.3389/fninf.2018.00087. eCollection 2018. Front Neuroinform. 2018. PMID: 30534067 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

