The alternative oxidase lowers mitochondrial reactive oxygen production in plant cells
- PMID: 10393984
- PMCID: PMC22224
- DOI: 10.1073/pnas.96.14.8271
The alternative oxidase lowers mitochondrial reactive oxygen production in plant cells
Abstract
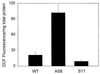
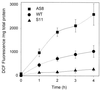
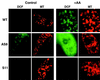
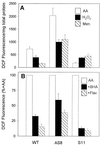
Besides the cytochrome c pathway, plant mitochondria have an alternative respiratory pathway that is comprised of a single homodimeric protein, alternative oxidase (AOX). Transgenic cultured tobacco cells with altered levels of AOX were used to test the hypothesis that the alternative pathway in plant mitochondria functions as a mechanism to decrease the formation of reactive oxygen species (ROS) produced during respiratory electron transport. Using the ROS-sensitive probe 2',7'-dichlorofluorescein diacetate, we found that antisense suppression of AOX resulted in cells with a significantly higher level of ROS compared with wild-type cells, whereas the overexpression of AOX resulted in cells with lower ROS abundance. Laser-scanning confocal microscopy showed that the difference in ROS abundance among wild-type and AOX transgenic cells was caused by changes in mitochondrial-specific ROS formation. Mitochondrial ROS production was exacerbated by the use of antimycin A, which inhibited normal cytochrome electron transport. In addition, cells overexpressing AOX were found to have consistently lower expression of genes encoding ROS-scavenging enzymes, including the superoxide dismutase genes SodA and SodB, as well as glutathione peroxidase. Also, the abundance of mRNAs encoding salicylic acid-binding catalase and a pathogenesis-related protein were significantly higher in cells deficient in AOX. These results are evidence that AOX plays a role in lowering mitochondrial ROS formation in plant cells.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

