UNC-11, a Caenorhabditis elegans AP180 homologue, regulates the size and protein composition of synaptic vesicles
- PMID: 10397769
- PMCID: PMC25452
- DOI: 10.1091/mbc.10.7.2343
UNC-11, a Caenorhabditis elegans AP180 homologue, regulates the size and protein composition of synaptic vesicles
Abstract
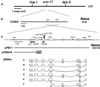
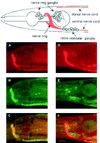
The unc-11 gene of Caenorhabditis elegans encodes multiple isoforms of a protein homologous to the mammalian brain-specific clathrin-adaptor protein AP180. The UNC-11 protein is expressed at high levels in the nervous system and at lower levels in other tissues. In neurons, UNC-11 is enriched at presynaptic terminals but is also present in cell bodies. unc-11 mutants are defective in two aspects of synaptic vesicle biogenesis. First, the SNARE protein synaptobrevin is mislocalized, no longer being exclusively localized to synaptic vesicles. The reduction of synaptobrevin at synaptic vesicles is the probable cause of the reduced neurotransmitter release observed in these mutants. Second, unc-11 mutants accumulate large vesicles at synapses. We propose that the UNC-11 protein mediates two functions during synaptic vesicle biogenesis: it recruits synaptobrevin to synaptic vesicle membranes and it regulates the size of the budded vesicle during clathrin coat assembly.
Figures

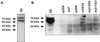






Similar articles
-
Regulation of neurotransmitter vesicles by the homeodomain protein UNC-4 and its transcriptional corepressor UNC-37/groucho in Caenorhabditis elegans cholinergic motor neurons.J Neurosci. 2001 Mar 15;21(6):2001-14. doi: 10.1523/JNEUROSCI.21-06-02001.2001. J Neurosci. 2001. PMID: 11245684 Free PMC article.
-
UNC-41/stonin functions with AP2 to recycle synaptic vesicles in Caenorhabditis elegans.PLoS One. 2012;7(7):e40095. doi: 10.1371/journal.pone.0040095. Epub 2012 Jul 10. PLoS One. 2012. PMID: 22808098 Free PMC article.
-
Ubiquitin and AP180 regulate the abundance of GLR-1 glutamate receptors at postsynaptic elements in C. elegans.Neuron. 2002 Jul 3;35(1):107-20. doi: 10.1016/s0896-6273(02)00749-3. Neuron. 2002. PMID: 12123612
-
Endocytosis: an assembly protein for clathrin cages.Curr Biol. 1999 May 6;9(9):R332-5. doi: 10.1016/s0960-9822(99)80206-1. Curr Biol. 1999. PMID: 10330371 Review.
-
The synaptic vesicle cycle: exocytosis and endocytosis in Drosophila and C. elegans.Curr Opin Neurobiol. 2002 Oct;12(5):499-507. doi: 10.1016/s0959-4388(02)00360-4. Curr Opin Neurobiol. 2002. PMID: 12367628 Review.
Cited by
-
Serum- and Glucocorticoid-Inducible Kinase-1 (SGK-1) Plays a Role in Membrane Trafficking in Caenorhabditis elegans.PLoS One. 2015 Jun 26;10(6):e0130778. doi: 10.1371/journal.pone.0130778. eCollection 2015. PLoS One. 2015. PMID: 26115433 Free PMC article.
-
NECAP 1 regulates AP-2 interactions to control vesicle size, number, and cargo during clathrin-mediated endocytosis.PLoS Biol. 2013 Oct;11(10):e1001670. doi: 10.1371/journal.pbio.1001670. Epub 2013 Oct 1. PLoS Biol. 2013. PMID: 24130457 Free PMC article.
-
Pharming for Genes in Neurotransmission: Combining Chemical and Genetic Approaches in Caenorhabditis elegans.ACS Chem Neurosci. 2018 Aug 15;9(8):1963-1974. doi: 10.1021/acschemneuro.7b00509. Epub 2018 Mar 6. ACS Chem Neurosci. 2018. PMID: 29432681 Free PMC article. Review.
-
Clathrin-mediated endocytosis is required for compensatory regulation of GLR-1 glutamate receptors after activity blockade.Proc Natl Acad Sci U S A. 2004 Mar 2;101(9):3190-5. doi: 10.1073/pnas.0306156101. Epub 2004 Feb 23. Proc Natl Acad Sci U S A. 2004. PMID: 14981253 Free PMC article.
-
Modeling Alzheimer's disease: from past to future.Front Pharmacol. 2013 Jun 19;4:77. doi: 10.3389/fphar.2013.00077. eCollection 2013. Front Pharmacol. 2013. PMID: 23801962 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

