Rho-dependent regulation of cell spreading by the tetraspan membrane protein Gas3/PMP22
- PMID: 10397775
- PMCID: PMC25466
- DOI: 10.1091/mbc.10.7.2441
Rho-dependent regulation of cell spreading by the tetraspan membrane protein Gas3/PMP22
Abstract
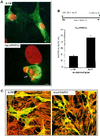
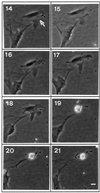
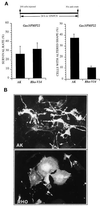
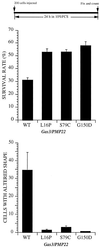
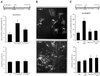
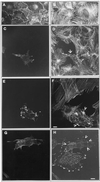
Gas3/PMP22 plays a crucial role in regulating myelin formation and maintenance, and different genetic alterations in gas3/PMP22 are responsible for a set of human peripheral neuropathies. We have previously demonstrated that Gas3/PMP22 could regulate susceptibility to apoptosis in NIH3T3 cells but not in REF 52 cells. In this report we demonstrate that when the apoptotic response triggered by gas3/PMP22 was counteracted by Bcl-2 coexpression, morphological changes were observed. Time-lapse analysis confirmed that Gas3/PMP22 can modulate cell spreading, and this effect was strengthened after inhibition of phosphoinositide 3-kinase. Using the active form of the small GTPase RhoA, we have been able to dissect the different Gas3/PMP22 biological activities. RhoA counteracted the Gas3/PMP22-dependent morphological response but was unable to neutralize the apoptotic response. Treatment of NIH3T3 cells with cytotoxic necrotizing factor 1, which activates endogenous Rho, also counteracted Gas3/PMP22-mediated cell shape and spreading changes. Treatment of REF 52 cells, which are unresponsive to Gas3/PMP22 overexpression, with the C3 exoenzyme, inhibiting Rho activity, renders REF 52 cells responsive to Gas3/PMP22 overexpression for cell shape and spreading changes. Finally, assembly of stress fibers and focal adhesions complexes, in response to lysophosphatidic acid-induced endogenous Rho activation, was impaired in Gas3/PMP22-overexpressing cells. We hypothesize that cell shape and spreading regulated by Gas3/PMP22 through the Rho GTPase might have an important role during Schwann cells differentiation and myelinization.
Figures




Similar articles
-
Alterations in the Arf6-regulated plasma membrane endosomal recycling pathway in cells overexpressing the tetraspan protein Gas3/PMP22.J Cell Sci. 2003 Mar 15;116(Pt 6):987-99. doi: 10.1242/jcs.00326. J Cell Sci. 2003. PMID: 12584243
-
Exposure at the cell surface is required for gas3/PMP22 To regulate both cell death and cell spreading: implication for the Charcot-Marie-Tooth type 1A and Dejerine-Sottas diseases.Mol Biol Cell. 2000 Sep;11(9):2901-14. doi: 10.1091/mbc.11.9.2901. Mol Biol Cell. 2000. PMID: 10982389 Free PMC article.
-
Apoptotic phenotype induced by overexpression of wild-type gas3/PMP22: its relation to the demyelinating peripheral neuropathy CMT1A.Genes Dev. 1995 Aug 1;9(15):1846-56. doi: 10.1101/gad.9.15.1846. Genes Dev. 1995. PMID: 7649472
-
Regulation of myelin-specific gene expression. Relevance to CMT1.Ann N Y Acad Sci. 1999 Sep 14;883:91-108. Ann N Y Acad Sci. 1999. PMID: 10586235 Review.
-
Roles for PMP22 in Schwann cell cholesterol homeostasis in health and disease.Biochem Soc Trans. 2024 Aug 28;52(4):1747-1756. doi: 10.1042/BST20231359. Biochem Soc Trans. 2024. PMID: 38979632 Free PMC article. Review.
Cited by
-
Peripheral myelin protein 22 is in complex with alpha6beta4 integrin, and its absence alters the Schwann cell basal lamina.J Neurosci. 2006 Jan 25;26(4):1179-89. doi: 10.1523/JNEUROSCI.2618-05.2006. J Neurosci. 2006. PMID: 16436605 Free PMC article.
-
MP20, the second most abundant lens membrane protein and member of the tetraspanin superfamily, joins the list of ligands of galectin-3.BMC Cell Biol. 2001;2:17. doi: 10.1186/1471-2121-2-17. Epub 2001 Aug 14. BMC Cell Biol. 2001. PMID: 11532191 Free PMC article.
-
Simultaneous and independent tuning of RhoA and Rac1 activity with orthogonally inducible promoters.Integr Biol (Camb). 2014 Sep;6(9):885-94. doi: 10.1039/c4ib00099d. Integr Biol (Camb). 2014. PMID: 25044255 Free PMC article.
-
Uncoupling of myelin assembly and schwann cell differentiation by transgenic overexpression of peripheral myelin protein 22.J Neurosci. 2000 Jun 1;20(11):4120-8. doi: 10.1523/JNEUROSCI.20-11-04120.2000. J Neurosci. 2000. PMID: 10818147 Free PMC article.
-
Unraveling the structures, functions and mechanisms of epithelial membrane protein family in human cancers.Exp Hematol Oncol. 2022 Oct 10;11(1):69. doi: 10.1186/s40164-022-00321-x. Exp Hematol Oncol. 2022. PMID: 36217151 Free PMC article. Review.
References
-
- Adlkofer K, Martini R, Aguzzi A, Zielasek J, Toyka KV, Suter U. Hypermyelination and demyelinating peripheral neuropathy in PMP22-deficient mice. Nat Genet. 1995;11:274–280. - PubMed
-
- Amano M, Chiahara K, Kimura K, Fukuta Y, Nakamura N, Matsuura Y, Kaibuchi K. Formation of actin stress fibers and focal adhesions is enhanced by Rho kinase. Science. 1997;275:1308–1311. - PubMed
-
- Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase) J Biol Chem. 1996;271:20246–20249. - PubMed
-
- Archelos JJ, Roggenbuck K, Schneider-Schaulies J, Linington C, Toyka KV, Hartung HP. Production and characterization of monoclonal antibodies to the extracellular domain of P0. J Neurosci Res. 1993;35:46–53. - PubMed
-
- Baechner D, Liehr T, Hameister H, Altenberger H, Grehl H, Suter U, Rautenstrauss B. Widespread expression of the peripheral myelin protein-22 gene (pmp22) in neural and nonneural tissue during murine development. J Neurosci Res. 1995;42:733–741. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

