Intranasal antibody prophylaxis for protection against viral disease
- PMID: 10398671
- PMCID: PMC100244
- DOI: 10.1128/CMR.12.3.383
Intranasal antibody prophylaxis for protection against viral disease
Abstract
For more than a century, antibody has been used for passive parenteral immunization against viral and bacterial pathogens. This approach has been successful for prevention of viral respiratory infection and has led to testing of intranasal or aerosol delivery of antibody to passively immunize the respiratory tract mucosal surface. Mucosal delivery may be advantageous because it allows the antibody to neutralize the virus particles before they initiate infection and because it concentrates the antibody where viral replication takes place. Animal studies have shown the feasibility of passive intranasal immunization against a number of respiratory tract viruses. Development of nasal antibody treatments for humans is under way, and early clinical studies have confirmed that this approach is safe and can be used to prevent respiratory tract disease. Polyclonal human immunoglobulin from pooled plasma preparations can be used to provide broad protection against a number of different pathogens, while monoclonal antibodies or their fragments can be used to target specific viruses.
Figures

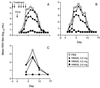
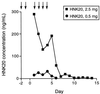
References
-
- Agius G, Dindinaud G, Biggar R J, Peyre R, Vaillant V, Ranger S, Poupet J Y, Cisse M F, Castets M. An epidemic of respiratory syncytial virus in elderly people: clinical and serological findings. J Med Virol. 1990;30:117–127. - PubMed
-
- Akerfeldt S, Geijer S, Holubars E, Fuchs G, Brundin M. Prophylactic and therapeutic antiviral effect of human gamma globulin. Biochem Pharmacol. 1972;21:503–509. - PubMed
-
- Akerfeldt S, Lofberg E, Fuchs G. Antiviral effect of human gamma globulin in mice. Comparison between the efficacy of local and systemic administration. Biochem Pharmacol. 1973;22:2911–2917. - PubMed
-
- Bansal G P, Hatfield J, Young J F, Top F H, Prince G A, Horswood R L, Hemming V, Hensen S. Efficacy of passively administered monoclonal antibodies against respiratory syncytial virus infection in cotton rats. Vaccines. 1991;91:283–288.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

