Heat-shock-induced activation of stress MAP kinase is regulated by threonine- and tyrosine-specific phosphatases
- PMID: 10398679
- PMCID: PMC316851
- DOI: 10.1101/gad.13.13.1653
Heat-shock-induced activation of stress MAP kinase is regulated by threonine- and tyrosine-specific phosphatases
Abstract
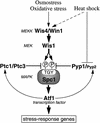
In eukaryotic species from yeast to human, stress-activated protein kinases (SAPKs), members of a MAP kinase (MAPK) subfamily, regulate the transcriptional response to various environmental stress. It is poorly understood how diverse forms of stress are sensed and transmitted to SAPKs. Here, we report the heat shock regulation of the fission yeast Spc1 SAPK, a homolog of human p38 and budding yeast Hog1p. Although osmostress and oxidative stress induce strong activation of the Wis1 MAPK kinase (MEK), which activates Spc1 through Thr-171/Tyr-173 phosphorylation, activation of Wis1 upon heat shock is relatively weak and transient. However, in heat-shocked cells, Pyp1, the major tyrosine phosphatase that dephosphorylates and inactivates Spc1, is inhibited for its interaction with Spc1, which leads to strong activation of Spc1. Subsequently, Spc1 activity is rapidly attenuated by Thr-171 dephosphorylation, whereas Tyr-173 remains phosphorylated. Thr-171 dephosphorylation is compromised in a strain lacking functional type 2C serine/threonine phosphatases (PP2C), Ptc1 and Ptc3. Moreover, Ptc1 and Ptc3 can dephosphorylate Thr-171 of Spc1 both in vivo and in vitro. These observations strongly suggest that PP2C enzymes play an important role in the attenuation of Spc1 activity in heat-shocked cells. Thus, transient activation of Spc1 upon heat shock is ensured by differential regulation of threonine and tyrosine phosphorylation.
Figures










Similar articles
-
Protein phosphatase 2C acts independently of stress-activated kinase cascade to regulate the stress response in fission yeast.J Biol Chem. 1997 Jul 11;272(28):17873-9. doi: 10.1074/jbc.272.28.17873. J Biol Chem. 1997. PMID: 9211944
-
Heat stress activates fission yeast Spc1/StyI MAPK by a MEKK-independent mechanism.Mol Biol Cell. 1998 Jun;9(6):1339-49. doi: 10.1091/mbc.9.6.1339. Mol Biol Cell. 1998. PMID: 9614178 Free PMC article.
-
Activation and regulation of the Spc1 stress-activated protein kinase in Schizosaccharomyces pombe.Mol Cell Biol. 1996 Jun;16(6):2870-7. doi: 10.1128/MCB.16.6.2870. Mol Cell Biol. 1996. PMID: 8649397 Free PMC article.
-
SAPKs and transcription factors do the nucleocytoplasmic tango.Genes Dev. 1998 May 15;12(10):1391-7. doi: 10.1101/gad.12.10.1391. Genes Dev. 1998. PMID: 9585499 Review. No abstract available.
-
Deciphering the MAP kinase pathway.Prog Growth Factor Res. 1994;5(3):291-334. doi: 10.1016/0955-2235(94)90011-6. Prog Growth Factor Res. 1994. PMID: 7888635 Review.
Cited by
-
Expression of hsp16 in response to nucleotide depletion is regulated via the spc1 MAPK pathway in Schizosaccharomyces pombe.Nucleic Acids Res. 2001 Jul 15;29(14):3030-40. doi: 10.1093/nar/29.14.3030. Nucleic Acids Res. 2001. PMID: 11452028 Free PMC article.
-
Identification of a Saccharomyces cerevisiae gene that is required for G1 arrest in response to the lipid oxidation product linoleic acid hydroperoxide.Mol Biol Cell. 2001 Jun;12(6):1801-10. doi: 10.1091/mbc.12.6.1801. Mol Biol Cell. 2001. PMID: 11408586 Free PMC article.
-
The Fission Yeast Cell Integrity Pathway: A Functional Hub for Cell Survival upon Stress and Beyond.J Fungi (Basel). 2021 Dec 30;8(1):32. doi: 10.3390/jof8010032. J Fungi (Basel). 2021. PMID: 35049972 Free PMC article. Review.
-
A Redox-Sensitive Thiol in Wis1 Modulates the Fission Yeast Mitogen-Activated Protein Kinase Response to H2O2 and Is the Target of a Small Molecule.Mol Cell Biol. 2020 Mar 16;40(7):e00346-19. doi: 10.1128/MCB.00346-19. Print 2020 Mar 16. Mol Cell Biol. 2020. PMID: 31932483 Free PMC article.
-
Dynamic regulation of Cdr1 kinase localization and phosphorylation during osmotic stress.J Biol Chem. 2017 Nov 10;292(45):18457-18468. doi: 10.1074/jbc.M117.793034. Epub 2017 Sep 18. J Biol Chem. 2017. PMID: 28924043 Free PMC article.
References
-
- Alessi DR, Gómez N, Moorhead G, Lewis T, Keyse SM, Cohen P. Inactivation of p42 MAP kinase by protein phosphatase 2A and a protein tyrosine phosphatase, but not CL100, in various cell lines. Curr Biol. 1995;5:283–295. - PubMed
-
- Alfa C, Fantes P, Hyams J, McLeod M, Warbrick E. Experiments with fission yeast: A laboratory course manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1993.
-
- Brewster JL, de Valoir T, Dwyer ND, Winter E, Gustin MC. An osmosensing signal transduction pathway in yeast. Science. 1993;259:1760–1763. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
