Xanthophyll cycle pigment localization and dynamics during exposure to low temperatures and light stress in vinca major
- PMID: 10398707
- PMCID: PMC59310
- DOI: 10.1104/pp.120.3.727
Xanthophyll cycle pigment localization and dynamics during exposure to low temperatures and light stress in vinca major
Abstract
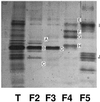
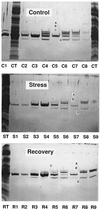
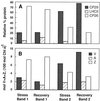
The distribution of xanthophyll cycle pigments (violaxanthin plus antheraxanthin plus zeaxanthin [VAZ]) among photosynthetic pigment-protein complexes was examined in Vinca major before, during, and subsequent to a photoinhibitory treatment at low temperature. Four pigment-protein complexes were isolated: the core of photosystem (PS) II, the major light-harvesting complex (LHC) protein of PSII (LHCII), the minor light-harvesting proteins (CPs) of PSII (CP29, CP26, and CP24), and PSI with its LHC proteins (PSI-LHCI). In isolated thylakoids 80% of VAZ was bound to protein independently of the de-epoxidation state and was found in all complexes. Plants grown outside in natural sunlight had higher levels of VAZ (expressed per chlorophyll), compared with plants grown in low light in the laboratory, and the additional VAZ was mainly bound to the major LHCII complex, apparently in an acid-labile site. The extent of de-epoxidation of VAZ in high light and the rate of reconversion of Z plus A to V following 2.5 h of recovery were greatest in the free-pigment fraction and varied among the pigment-protein complexes. Photoinhibition caused increases in VAZ, particularly in low-light-acclimated leaves. The data suggest that the photoinhibitory treatment caused an enrichment in VAZ bound to the minor CPs caused by de novo synthesis of the pigments and/or a redistribution of VAZ from the major LHCII complex.
Figures
References
-
- Adams WW, III, Demmig-Adams B, Verhoeven AS, Barker DH. 'Photoinhibition' during winter stress: involvement of sustained xanthophyll cycle-dependent energy dissipation. Aust J Plant Physiol. 1995;22:261–276.
-
- Adamska I. ELIPs: light-induced stress proteins. Physiol Plant. 1997;100:794–805.
-
- Bassi R, Dainese P. A supramolecular light-harvesting complex from chloroplast photosystem-II membranes. Eur J Biochem. 1992;204:317–326. - PubMed
-
- Bassi R, Giacometti GM, Simpson D. Changes in the composition of stroma lamellae following state I–state II transitions. Biochim Biophys Acta. 1988;935:152–165.
-
- Bassi R, Hinz U, Barbato R. The role of light harvesting complex and photosystem II in thylakoid stacking in the chlorina-f2 barley mutant. Carlsberg Res Commun. 1985;50:347–367.
LinkOut - more resources
Full Text Sources

