Lysophosphatidic acid acyltransferase from coconut endosperm mediates the insertion of laurate at the sn-2 position of triacylglycerols in lauric rapeseed oil and can increase total laurate levels
- PMID: 10398708
- PMCID: PMC59311
- DOI: 10.1104/pp.120.3.739
Lysophosphatidic acid acyltransferase from coconut endosperm mediates the insertion of laurate at the sn-2 position of triacylglycerols in lauric rapeseed oil and can increase total laurate levels
Abstract
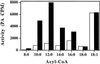
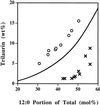
Expression of a California bay laurel (Umbellularia californica) 12:0-acyl-carrier protein thioesterase, bay thioesterase (BTE), in developing seeds of oilseed rape (Brassica napus) led to the production of oils containing up to 50% laurate. In these BTE oils, laurate is found almost exclusively at the sn-1 and sn-3 positions of the triacylglycerols (T.A. Voelker, T.R. Hayes, A.C. Cranmer, H.M. Davies [1996] Plant J 9: 229-241). Coexpression of a coconut (Cocos nucifera) 12:0-coenzyme A-preferring lysophosphatitic acid acyltransferase (D.S. Knutzon, K.D. Lardizabal, J.S. Nelsen, J.L. Bleibaum, H.M. Davies, J.G. Metz [1995] Plant Physiol 109: 999-1006) in BTE oilseed rape seeds facilitates efficient laurate deposition at the sn-2 position, resulting in the acccumulation of trilaurin. The introduction of the coconut protein into BTE oilseed rape lines with laurate above 50 mol % further increases total laurate levels.
Figures




Similar articles
-
Fatty acid distribution and lipid metabolism in developing seeds of laurate-producing rape (Brassica napus L.).Planta. 1997;203(3):341-8. doi: 10.1007/s004250050200. Planta. 1997. PMID: 9431681
-
The distribution of caprylate, caprate and laurate in lipids from developing and mature seeds of transgenic Brassica napus L.Planta. 2000 Dec;212(1):33-40. doi: 10.1007/s004250000361. Planta. 2000. PMID: 11219581
-
Expression of lauroyl-acyl carrier protein thioesterase in brassica napus seeds induces pathways for both fatty acid oxidation and biosynthesis and implies a set point for triacylglycerol accumulation.Plant Cell. 1998 Apr;10(4):613-22. doi: 10.1105/tpc.10.4.613. Plant Cell. 1998. PMID: 9548986 Free PMC article.
-
Rapeseed species and environmental concerns related to loss of seeds of genetically modified oilseed rape in Japan.GM Crops. 2010 May-Jun;1(3):143-56. doi: 10.4161/gmcr.1.3.12761. GM Crops. 2010. PMID: 21844669 Review.
-
Genetic and molecular approaches to improve nutritional value of Brassica napus L. seed.C R Biol. 2008 Oct;331(10):763-71. doi: 10.1016/j.crvi.2008.07.018. Epub 2008 Sep 4. C R Biol. 2008. PMID: 18926490 Review.
Cited by
-
A distinct DGAT with sn-3 acetyltransferase activity that synthesizes unusual, reduced-viscosity oils in Euonymus and transgenic seeds.Proc Natl Acad Sci U S A. 2010 May 18;107(20):9464-9. doi: 10.1073/pnas.1001707107. Epub 2010 May 3. Proc Natl Acad Sci U S A. 2010. PMID: 20439724 Free PMC article.
-
Metabolic engineering of medium-chain fatty acid biosynthesis in Nicotiana benthamiana plant leaf lipids.Front Plant Sci. 2015 Mar 24;6:164. doi: 10.3389/fpls.2015.00164. eCollection 2015. Front Plant Sci. 2015. PMID: 25852716 Free PMC article.
-
Vegetable Oil: Nutritional and Industrial Perspective.Curr Genomics. 2016 Jun;17(3):230-40. doi: 10.2174/1389202917666160202220107. Curr Genomics. 2016. PMID: 27252590 Free PMC article.
-
Expression of rapeseed microsomal lysophosphatidic acid acyltransferase isozymes enhances seed oil content in Arabidopsis.Plant Physiol. 2010 Feb;152(2):670-84. doi: 10.1104/pp.109.148247. Epub 2009 Dec 4. Plant Physiol. 2010. PMID: 19965969 Free PMC article.
-
De Novo Genome Sequence Assembly of Dwarf Coconut (Cocos nucifera L. 'Catigan Green Dwarf') Provides Insights into Genomic Variation Between Coconut Types and Related Palm Species.G3 (Bethesda). 2019 Aug 8;9(8):2377-2393. doi: 10.1534/g3.119.400215. G3 (Bethesda). 2019. PMID: 31167834 Free PMC article.
References
-
- Bafor M, Stobart AK, Stymne S. Properties of the glycerol acylating enzymes in microsomal preparations from the developing seeds of safflower (Carthamus tinctorius) and turnip rape (Brassica campestris) and their ability to assemble cocoa-butter type fats. J Am Oil Chem Soc. 1990;67:217–225.
-
- Bernatzky R, Tanksley SD. Genetics of actin-related sequences in tomato. Theor Appl Genet. 1986;72:314–321. - PubMed
-
- Browse J, McCourt J, Somerville CR. Fatty acid composition of leaf lipids determined after combined digestion and fatty acid methyl ester formation from fresh tissue. Anal Biochem. 1986;152:141–145. - PubMed
-
- Davies HM, Hawkins DJ, Nelsen JS. Lysophosphatidic acid acyltransferase from immature coconut endosperm having medium chain length substrate specificity. Phytochemistry. 1995;39:989–996.
-
- Del Vecchio AJD. High laurate canola. Inform. 1996;7:230–242.
LinkOut - more resources
Full Text Sources
Other Literature Sources

