Limonene synthase, the enzyme responsible for monoterpene biosynthesis in peppermint, is localized to leucoplasts of oil gland secretory cells
- PMID: 10398724
- PMCID: PMC59327
- DOI: 10.1104/pp.120.3.879
Limonene synthase, the enzyme responsible for monoterpene biosynthesis in peppermint, is localized to leucoplasts of oil gland secretory cells
Abstract
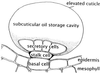
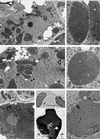
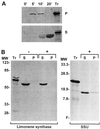
Circumstantial evidence based on ultrastructural correlation, specific labeling, and subcellular fractionation studies indicates that at least the early steps of monoterpene biosynthesis occur in plastids. (4S)-Limonene synthase, which is responsible for the first dedicated step of monoterpene biosynthesis in mint species, appears to be translated as a preprotein bearing a long plastidial transit peptide. Immunogold labeling using polyclonal antibodies raised to the native enzyme demonstrated the specific localization of limonene synthase to the leucoplasts of peppermint (Mentha x piperita) oil gland secretory cells during the period of essential oil production. Labeling was shown to be absent from all other plastid types examined, including the basal and stalk cell plastids of the secretory phase glandular trichomes. Furthermore, in vitro translation of the preprotein and import experiments with isolated pea chloroplasts were consistent in demonstrating import of the nascent protein to the plastid stroma and proteolytic processing to the mature enzyme at this site. These experiments confirm that the leucoplastidome of the oil gland secretory cells is the exclusive location of limonene synthase, and almost certainly the preceding steps of monoterpene biosynthesis, in peppermint leaves. However, succeeding steps of monoterpene metabolism in mint appear to occur outside the leucoplasts of oil gland cells.
Figures
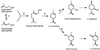
References
-
- Adam K-P, Theil R, Zapp J, Becker H. Involvement of the mevalonic acid pathway and the glyceraldehyde-pyruvate pathway in terpenoid biosynthesis of the liverworts Ricciocarpos natans, and Conocephalum conicum. Arch Biochem Biophys. 1998;354:181–187. - PubMed
-
- Alonso WR, Crock JE, Croteau R. Production and characterization of polyclonal antibodies in rabbits to 4S-limonene synthase from spearmint (Mentha spicata) Arch Biochem Biophys. 1993;301:58–63. - PubMed
-
- Alonso WR, Rajaonarivony JIM, Gershenzon J, Croteau R. Purification of 4S-limonene synthase, a monoterpene cyclase from the glandular trichomes of peppermint (Mentha × piperita) and spearmint (M. spicata) J Biol Chem. 1992;267:7582–7587. - PubMed
-
- Amelunxen F. Electromikroskopishe Untersuchungen an den Drüsenschuppen von Mentha piperita L. Planta Med. 1965;13:457–473.
-
- Amelunxen F, Wahlig T, Arbeiter H. Über den Nachweis des atherischen Öls in isolierten Drüsenhaaren und Drüsenschuppen von Mentha piperita L. Z Pflanzenphysiol. 1969;61:68–72.
LinkOut - more resources
Full Text Sources
Other Literature Sources

