Species-independent inhibition of abnormal prion protein (PrP) formation by a peptide containing a conserved PrP sequence
- PMID: 10400714
- PMCID: PMC112701
- DOI: 10.1128/JVI.73.8.6245-6250.1999
Species-independent inhibition of abnormal prion protein (PrP) formation by a peptide containing a conserved PrP sequence
Abstract
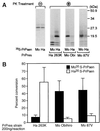
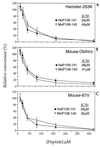
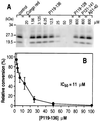
Conversion of the normal protease-sensitive prion protein (PrP) to its abnormal protease-resistant isoform (PrP-res) is a major feature of the pathogenesis associated with transmissible spongiform encephalopathy (TSE) diseases. In previous experiments, PrP conversion was inhibited by a peptide composed of hamster PrP residues 109 to 141, suggesting that this region of the PrP molecule plays a crucial role in the conversion process. In this study, we used PrP-res derived from animals infected with two different mouse scrapie strains and one hamster scrapie strain to investigate the species specificity of these conversion reactions. Conversion of PrP was found to be completely species specific; however, despite having three amino acid differences, peptides corresponding to the hamster and mouse PrP sequences from residues 109 to 141 inhibited both the mouse and hamster PrP conversion systems equally. Furthermore, a peptide corresponding to hamster PrP residues 119 to 136, which was identical in both mouse and hamster PrP, was able to inhibit PrP-res formation in both the mouse and hamster cell-free systems as well as in scrapie-infected mouse neuroblastoma cell cultures. Because the PrP region from 119 to 136 is very conserved in most species, this peptide may have inhibitory effects on PrP conversion in a wide variety of TSE diseases.
Figures

Similar articles
-
A single hamster PrP amino acid blocks conversion to protease-resistant PrP in scrapie-infected mouse neuroblastoma cells.J Virol. 1995 Dec;69(12):7754-8. doi: 10.1128/JVI.69.12.7754-7758.1995. J Virol. 1995. PMID: 7494285 Free PMC article.
-
Flexible N-terminal region of prion protein influences conformation of protease-resistant prion protein isoforms associated with cross-species scrapie infection in vivo and in vitro.J Biol Chem. 2004 Apr 2;279(14):13689-95. doi: 10.1074/jbc.M303697200. Epub 2004 Jan 21. J Biol Chem. 2004. PMID: 14736880
-
Species specificity in the cell-free conversion of prion protein to protease-resistant forms: a model for the scrapie species barrier.Proc Natl Acad Sci U S A. 1995 Apr 25;92(9):3923-7. doi: 10.1073/pnas.92.9.3923. Proc Natl Acad Sci U S A. 1995. PMID: 7732006 Free PMC article.
-
Prion protein and species barriers in the transmissible spongiform encephalopathies.Biomed Pharmacother. 1999;53(1):27-33. doi: 10.1016/s0753-3322(99)80057-2. Biomed Pharmacother. 1999. PMID: 10221165 Review.
-
Molecular biology of prions causing infectious and genetic encephalopathies of humans as well as scrapie of sheep and BSE of cattle.Dev Biol Stand. 1991;75:55-74. Dev Biol Stand. 1991. PMID: 1686599 Review.
Cited by
-
Evidence of a molecular barrier limiting susceptibility of humans, cattle and sheep to chronic wasting disease.EMBO J. 2000 Sep 1;19(17):4425-30. doi: 10.1093/emboj/19.17.4425. EMBO J. 2000. PMID: 10970836 Free PMC article.
-
A naturally occurring C-terminal fragment of the prion protein (PrP) delays disease and acts as a dominant-negative inhibitor of PrPSc formation.J Biol Chem. 2011 Dec 23;286(51):44234-44242. doi: 10.1074/jbc.M111.286195. Epub 2011 Oct 24. J Biol Chem. 2011. PMID: 22025612 Free PMC article.
-
Design and Optimization of Anti-amyloid Domain Antibodies Specific for β-Amyloid and Islet Amyloid Polypeptide.J Biol Chem. 2016 Feb 5;291(6):2858-73. doi: 10.1074/jbc.M115.682336. Epub 2015 Nov 24. J Biol Chem. 2016. PMID: 26601942 Free PMC article.
-
PrP(C) association with lipid rafts in the early secretory pathway stabilizes its cellular conformation.Mol Biol Cell. 2004 Sep;15(9):4031-42. doi: 10.1091/mbc.e03-05-0271. Epub 2004 Jun 30. Mol Biol Cell. 2004. PMID: 15229281 Free PMC article.
-
Recent advances in prion chemotherapeutics.Infect Disord Drug Targets. 2009 Feb;9(1):81-91. doi: 10.2174/1871526510909010081. Infect Disord Drug Targets. 2009. PMID: 19200018 Free PMC article. Review.
References
-
- Brandner S, Isenmann S, Raeber A, Fischer M, Sailer A, Kobayashi Y, Marino S, Weissmann C, Aguzzi A. Normal host prion protein necessary for scrapie-induced neurotoxicity. Nature. 1996;379:339–343. - PubMed
-
- Bruce M E, McConnell I, Fraser H, Dickinson A G. The disease characteristics of different strains of scrapie in Sinc congenic mouse lines: implications for the nature of the agent and host control of pathogenesis. J Gen Virol. 1991;72:595–603. - PubMed
-
- Caughey B. Protease-resistant PrP accumulation and scrapie agent replication: a role for sulphated glycosaminoglycans? Biochem Soc Trans. 1994;22:163–167. - PubMed
-
- Caughey B, Chesebro B. Prion protein and the transmissible spongiform encephalopathies. Trends Cell Biol. 1997;7:56–62. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

