Attenuated, replication-competent herpes simplex virus type 1 mutant G207: safety evaluation of intracerebral injection in nonhuman primates
- PMID: 10400723
- PMCID: PMC112710
- DOI: 10.1128/JVI.73.8.6319-6326.1999
Attenuated, replication-competent herpes simplex virus type 1 mutant G207: safety evaluation of intracerebral injection in nonhuman primates
Abstract
This study examined the safety of intracerebral inoculation of G207, an attenuated, replication-competent herpes simplex virus type 1 (HSV-1) recombinant, in nonhuman primates. Sixteen New World owl monkeys (Aotus nancymae [karyotype 1, formerly believed to be A. trivirgatus]), known for their exquisite susceptibility to HSV-1 infection, were evaluated. Thirteen underwent intracerebral inoculation with G207 at doses of 10(7) or 10(9) PFU, two were vehicle inoculated, and one served as an infected wild-type control and received 10(3) PFU of HSV-1 strain F. HSV-1 strain F caused rapid mortality and symptoms consistent with HSV encephalitis, including fever, hemiparesis, meningitis, and hemorrhage in the basal ganglia. One year after G207 inoculation, seven of the animals were alive and exhibited no evidence of clinical complications. Three deaths resulted from nonneurologic causes unrelated to HSV infection, and three animals were sacrificed for histopathologic examination. Two animals were reinoculated with G207 (10(7) PFU) at the same stereotactic coordinates 1 year after the initial G207 inoculation. These animals were alive and healthy 2 years after the second inoculation. Cerebral magnetic resonance imaging studies performed both before and after G207 inoculation failed to reveal radiographic evidence of HSV-related sequelae. Despite the lack of outwardly observable HSV pathology, measurable increases in serum anti-HSV titers were detected. Histopathological examination of multiple organ tissues found no evidence of HSV-induced histopathology or dissemination. We conclude that intracerebral inoculation of up to 10(9) PFU of G207, well above the efficacious dose in mouse tumor studies, is safe and therefore appropriate for human clinical trials.
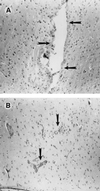
Figures




Similar articles
-
Viral shedding and biodistribution of G207, a multimutated, conditionally replicating herpes simplex virus type 1, after intracerebral inoculation in aotus.Mol Ther. 2000 Dec;2(6):588-95. doi: 10.1006/mthe.2000.0200. Mol Ther. 2000. PMID: 11124059
-
Attenuated, replication-competent herpes simplex virus type 1 mutant G207: safety evaluation in mice.J Virol. 2000 Apr;74(8):3832-41. doi: 10.1128/jvi.74.8.3832-3841.2000. J Virol. 2000. PMID: 10729157 Free PMC article.
-
Preclinical safety evaluation of G207, a replication-competent herpes simplex virus type 1, inoculated intraprostatically in mice and nonhuman primates.Hum Gene Ther. 2001 May 20;12(8):999-1010. doi: 10.1089/104303401750195944. Hum Gene Ther. 2001. PMID: 11387063
-
Immuno-viral therapy as a new approach for the treatment of brain tumors.Drug News Perspect. 2003 May;16(4):223-9. doi: 10.1358/dnp.2003.16.4.829334. Drug News Perspect. 2003. PMID: 12942152 Review.
-
Oncolytic virus therapy using genetically engineered herpes simplex viruses.Front Biosci. 2008 Jan 1;13:2060-4. doi: 10.2741/2823. Front Biosci. 2008. PMID: 17981691 Review.
Cited by
-
Oncolytic viral therapy for human prostate cancer by conditionally replicating herpes simplex virus 1 vector G207.Jpn J Cancer Res. 2000 Dec;91(12):1339-44. doi: 10.1111/j.1349-7006.2000.tb00923.x. Jpn J Cancer Res. 2000. PMID: 11123435 Free PMC article.
-
Ras signaling influences permissiveness of malignant peripheral nerve sheath tumor cells to oncolytic herpes.Am J Pathol. 2008 Dec;173(6):1861-72. doi: 10.2353/ajpath.2008.080376. Epub 2008 Nov 6. Am J Pathol. 2008. PMID: 18988803 Free PMC article.
-
Herpes simplex viruses: is a vaccine tenable?J Clin Invest. 2002 Jul;110(2):145-51. doi: 10.1172/JCI16126. J Clin Invest. 2002. PMID: 12122103 Free PMC article. Review. No abstract available.
-
Oncolytic polio virotherapy of cancer.Cancer. 2014 Nov 1;120(21):3277-86. doi: 10.1002/cncr.28862. Epub 2014 Jun 17. Cancer. 2014. PMID: 24939611 Free PMC article. Review.
-
Stimulus-responsive viral vectors for controlled delivery of therapeutics.J Control Release. 2017 Dec 10;267:80-89. doi: 10.1016/j.jconrel.2017.08.021. Epub 2017 Aug 24. J Control Release. 2017. PMID: 28842318 Free PMC article. Review.
References
-
- Ashley R, Benedetti J, Corey L. Humoral immune response to HSV-1 and HSV-2 viral proteins in patients with primary genital herpes. J Med Virol. 1985;17:153–166. - PubMed
-
- Boviatsis E J, Scharf J M, Chase M, Harrington K, Kowall N W, Breakefield X O, Chiocca E A. Antitumor activity and reporter gene transfer into rat brain neoplasms inoculated with herpes simplex virus vectors defective in thymidine kinase or ribonucleotide reductase. Gene Ther. 1994;1:323–331. - PubMed
-
- Budka H, Popow-Kraupp T. Rabies and herpes simplex virus encephalitis. An immunohistological study on site and distribution of viral antigens. Virchows Arch Abt A. 1981;390:353–364. - PubMed
-
- Cameron J M, McDougall I, Marsden H S, Preston V G, Ryan D M, Subak-Sharpe J H. Ribonucleotide reductase encoded by herpes simplex virus is a determinant of the pathogenicity of the virus in mice and a valid antiviral target. J Gen Virol. 1988;69:2607–2612. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

