The Epstein-Barr virus BZLF1 protein interacts physically and functionally with the histone acetylase CREB-binding protein
- PMID: 10400751
- PMCID: PMC112738
- DOI: 10.1128/JVI.73.8.6551-6558.1999
The Epstein-Barr virus BZLF1 protein interacts physically and functionally with the histone acetylase CREB-binding protein
Abstract
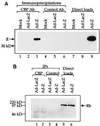
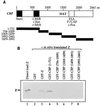
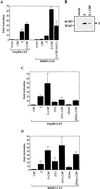
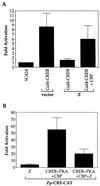
The Epstein-Barr virus (EBV) immediate-early protein BZLF1 (Z) is a key regulator of the EBV latent-to-lytic switch. Z is a transcriptional activator which induces EBV early gene expression. We demonstrate here that Z interacts with CREB-binding protein (CBP), a histone acetylase and transcriptional coactivator. This interaction requires the amino-terminal region of CBP as well as the transactivation and leucine zipper domains of Z. We show that CBP enhances Z-mediated transactivation of EBV early promoters, in reporter gene assays and in the context of the endogenous genome. We also demonstrate that Z decreases CREB transactivation function and that this inhibitory effect is reversed by overexpression of CBP. We show that Z also interacts directly with CREB. However, mutational analysis indicates that Z inhibition of CREB activity requires the direct interaction between Z and CBP but not the direct interaction between Z and CREB. We propose that Z interacts with CBP to enhance viral early gene transcription. In addition, the Z-CBP interaction may control host cellular transcription factor activity through competition for limiting amounts of cellular CBP.
Figures



References
-
- Arany Z, Newsome D, Oldread E, Livingston D M, Eckner R. A family of transcriptional adaptor proteins targeted by the E1A oncoprotein. Nature. 1995;374:81–84. - PubMed
-
- Bannister A J, Oehler T, Wilhelm D, Angel P, Kouzarides T. Stimulation of c-Jun activity by CBP: c-Jun residues Ser63/73 are required for CBP induced stimulation in vivo and CBP binding in vitro. Oncogene. 1995;11:2509–2514. - PubMed
-
- Bannister A J, Kouzarides T. The CBP co-activator is a histone acetylase. Nature. 1996;384:641–643. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

