Identification of a linear heparin binding domain for human respiratory syncytial virus attachment glycoprotein G
- PMID: 10400758
- PMCID: PMC112745
- DOI: 10.1128/JVI.73.8.6610-6617.1999
Identification of a linear heparin binding domain for human respiratory syncytial virus attachment glycoprotein G
Abstract
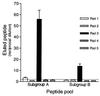
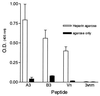
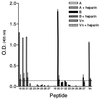
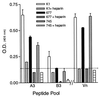
Respiratory syncytial virus (RSV) is the leading cause of lower respiratory tract disease in infants and young children worldwide. Infection is mediated, in part, by an initial interaction between attachment protein (G) and a highly sulfated heparin-like glycosaminoglycan (Gag) located on the cell surface. Synthetic overlapping peptides derived from consensus sequences of the G protein ectodomain from both RSV subgroups A and B were tested by heparin-agarose affinity chromatography for their abilities to bind heparin. This evaluation identified a single linear heparin binding domain (HBD) for RSV subgroup A (184A-->T198) and B (183K-->K197). The binding of these peptides to Vero cells was inhibited by heparin. Peptide binding to two CHO cell mutants (pgsD-677 and pgsA-745) deficient in heparan sulfate or total Gag synthesis was decreased 50% versus the parental cell line, CHO-K1, and decreased an average of 87% in the presence of heparin. The RSV-G HBD peptides were also able to inhibit homologous and heterologous virus infectivity of Vero cells. These results indicate that the sequence 184A/183K-->198T/K197 for RSV subgroups A and B, respectively, defines an important determinant of RSV-G interactions with heparin.
Figures

Similar articles
-
Identification of linear heparin-binding peptides derived from human respiratory syncytial virus fusion glycoprotein that inhibit infectivity.J Virol. 2007 Jan;81(1):261-71. doi: 10.1128/JVI.01226-06. Epub 2006 Oct 18. J Virol. 2007. PMID: 17050595 Free PMC article.
-
The fusion glycoprotein of human respiratory syncytial virus facilitates virus attachment and infectivity via an interaction with cellular heparan sulfate.J Virol. 2000 Jul;74(14):6442-7. doi: 10.1128/jvi.74.14.6442-6447.2000. J Virol. 2000. PMID: 10864656 Free PMC article.
-
Respiratory syncytial virus with the fusion protein as its only viral glycoprotein is less dependent on cellular glycosaminoglycans for attachment than complete virus.Virology. 2002 Mar 15;294(2):296-304. doi: 10.1006/viro.2001.1340. Virology. 2002. PMID: 12009871
-
Heparin-dependent attachment of respiratory syncytial virus (RSV) to host cells.Arch Virol. 1997;142(6):1247-54. doi: 10.1007/s007050050156. Arch Virol. 1997. PMID: 9229012
-
Binding and entry of respiratory syncytial virus into host cells and initiation of the innate immune response.Cell Microbiol. 2003 Oct;5(10):671-80. doi: 10.1046/j.1462-5822.2003.00313.x. Cell Microbiol. 2003. PMID: 12969373 Review.
Cited by
-
CX3CR1 Is Expressed in Differentiated Human Ciliated Airway Cells and Co-Localizes with Respiratory Syncytial Virus on Cilia in a G Protein-Dependent Manner.PLoS One. 2015 Jun 24;10(6):e0130517. doi: 10.1371/journal.pone.0130517. eCollection 2015. PLoS One. 2015. PMID: 26107373 Free PMC article.
-
The respiratory syncytial virus G protein conserved domain induces a persistent and protective antibody response in rodents.PLoS One. 2012;7(3):e34331. doi: 10.1371/journal.pone.0034331. Epub 2012 Mar 29. PLoS One. 2012. PMID: 22479601 Free PMC article.
-
Antiviral potential of natural products from marine microbes.Eur J Med Chem. 2020 Dec 1;207:112790. doi: 10.1016/j.ejmech.2020.112790. Epub 2020 Aug 31. Eur J Med Chem. 2020. PMID: 32937282 Free PMC article. Review.
-
Mammalian Cell-Derived Respiratory Syncytial Virus-Like Particles Protect the Lower as well as the Upper Respiratory Tract.PLoS One. 2015 Jul 14;10(7):e0130755. doi: 10.1371/journal.pone.0130755. eCollection 2015. PLoS One. 2015. PMID: 26172453 Free PMC article.
-
Respiratory syncytial virus fusion protein mediates inhibition of mitogen-induced T-cell proliferation by contact.J Virol. 2002 Feb;76(3):1163-70. doi: 10.1128/jvi.76.3.1163-1170.2002. J Virol. 2002. PMID: 11773392 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

