Expression of mouse mammary tumor virus superantigen mRNA in the thymus correlates with kinetics of self-reactive T-cell loss
- PMID: 10400761
- PMCID: PMC112748
- DOI: 10.1128/JVI.73.8.6634-6645.1999
Expression of mouse mammary tumor virus superantigen mRNA in the thymus correlates with kinetics of self-reactive T-cell loss
Abstract
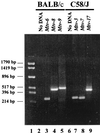
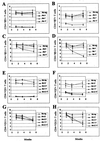
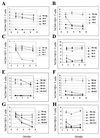
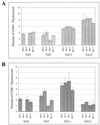
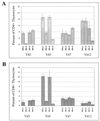
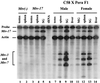
Mouse mammary tumor virus (MMTV) encodes a superantigen (Sag) that is expressed at the surface of antigen-presenting cells in conjunction with major histocompatibility complex (MHC) type II molecules. The Sag-MHC complex is recognized by entire subsets of T cells, leading to cytokine release and amplification of infected B and T cells that carry milk-borne MMTV to the mammary gland. Expression of Sag proteins from endogenous MMTV proviruses carried in the mouse germ line usually results in the deletion of self-reactive T cells during negative selection in the thymus and the elimination of T cells required for infection by specific milk-borne MMTVs. However, other endogenous MMTVs are unable to eliminate Sag-reactive T cells in newborn mice and cause partial loss of reactive T cells in adults. To investigate the kinetics of Sag-reactive T-cell deletion, backcross mice that contain single or multiple MMTVs were screened by a novel PCR assay designed to distinguish among highly related MMTV strains. Mice that contained Mtv-17 alone showed slow kinetics of reactive T-cell loss that involved the CD4(+), but not the CD8(+), subset. Deletion of CD4(+) or CD8(+) T cells reactive with Mtv-17 Sag was not detected in thymocytes. Slow kinetics of peripheral T-cell deletion by Mtv-17 Sag also was accompanied by failure to detect Mtv-17 sag-specific mRNA in the thymus, despite detectable expression in other tissues, such as spleen. Together, these data suggest that Mtv-17 Sag causes peripheral, rather than intrathymic, deletion of T cells. Interestingly, the Mtv-8 provirus caused partial deletion of CD4(+)Vbeta12(+) cells in the thymus, but other T-cell subsets appeared to be deleted only in the periphery. Our data have important implications for the level of antigen expression required for elimination of self-reactive T cells. Moreover, these experiments suggest that mice expressing endogenous MMTVs that lead to slow kinetics of T-cell deletion will be susceptible to infection by milk-borne MMTVs with the same Sag specificity.
Figures

Similar articles
-
Exogenous mouse mammary tumor virus (MMTV) infection induces endogenous MMTV sag expression.Virology. 1996 Jan 15;215(2):113-23. doi: 10.1006/viro.1996.0014. Virology. 1996. PMID: 8560758
-
Quantitation of endogenous mouse mammary tumor virus superantigen expression by lymphocyte subsets.Eur J Immunol. 1995 Sep;25(9):2632-7. doi: 10.1002/eji.1830250934. Eur J Immunol. 1995. PMID: 7589137
-
Strain-specific expression of spliced MMTV RNAs containing the superantigen gene.Virology. 1997 Sep 15;236(1):54-65. doi: 10.1006/viro.1997.8717. Virology. 1997. PMID: 9299617
-
Thymic repertoire selection by superantigens: presentation by human and mouse MHC molecules.Thymus. 1994;23(1):1-13. Thymus. 1994. PMID: 7863543 Review.
-
The role of superantigens in the immunobiology of retroviruses.Ciba Found Symp. 1994;187:132-40; discussion 140-3. doi: 10.1002/9780470514672.ch9. Ciba Found Symp. 1994. PMID: 7796668 Review.
Cited by
-
Sequence and genetic analyses of the 3' terminus and integration sites of the RIII/Sa mouse mammary tumor (MMTV) exogenous provirus.Virus Genes. 2001;23(1):35-43. doi: 10.1023/a:1011175112113. Virus Genes. 2001. PMID: 11556399
-
BALB/Mtv-null mice responding to strong mouse mammary tumor virus superantigens restrict mammary tumorigenesis.J Virol. 2009 Jan;83(1):484-8. doi: 10.1128/JVI.01374-08. Epub 2008 Oct 15. J Virol. 2009. PMID: 18922863 Free PMC article.
-
C3H mouse mammary tumor virus superantigen function requires a splice donor site in the envelope gene.J Virol. 2000 Oct;74(20):9431-40. doi: 10.1128/jvi.74.20.9431-9440.2000. J Virol. 2000. PMID: 11000212 Free PMC article.
-
Endogenous MMTV proviruses induce susceptibility to both viral and bacterial pathogens.PLoS Pathog. 2006 Dec;2(12):e128. doi: 10.1371/journal.ppat.0020128. PLoS Pathog. 2006. PMID: 17140288 Free PMC article.
-
Regulatory T-cell expansion during chronic viral infection is dependent on endogenous retroviral superantigens.Proc Natl Acad Sci U S A. 2011 Mar 1;108(9):3677-82. doi: 10.1073/pnas.1100213108. Epub 2011 Feb 14. Proc Natl Acad Sci U S A. 2011. PMID: 21321220 Free PMC article.
References
-
- Abe R, Foo-Phillips M, Granger L G, Kanagawa O. Characterization of the Mlsf system. I. A novel “polymorphism” of endogenous superantigens. J Immunol. 1992;149:3429–3439. - PubMed
-
- Abe R, Kanagawa O, Sheard M A, Malissen B, Foo-Phillips M. Characterization of a new minor lymphocyte stimulatory system. I. Cluster of self antigens recognized by “I-E-reactive” Vβs, Vβ5, Vβ11, and Vβ12 T cell receptors for antigen. J Immunol. 1991;147:739–749. - PubMed
-
- Acha-Orbea H, Held W, Waanders G A, Shakhov A N, Scarpellino L, Lees R K, MacDonald H R. Exogenous and endogenous mouse mammary tumor virus superantigens. Immunol Rev. 1993;131:5–25. - PubMed
-
- Acha-Orbea H, MacDonald H R. Superantigens of mouse mammary tumor virus. Annu Rev Immunol. 1995;13:459–486. - PubMed
-
- Acha-Orbea H, Shakhov A N, Scarpellino L, Kolb E, Muller V, Vessaz-Shaw A, Fuchs R, Blochlinger K, Rollini P, Billotte J, et al. Clonal deletion of Vβ14-bearing T cells in mice transgenic for mammary tumour virus. Nature. 1991;350:207–211. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

