Variability of human systemic humoral immune responses to adenovirus gene transfer vectors administered to different organs
- PMID: 10400771
- PMCID: PMC112758
- DOI: 10.1128/JVI.73.8.6729-6742.1999
Variability of human systemic humoral immune responses to adenovirus gene transfer vectors administered to different organs
Abstract
Administration of adenovirus (Ad) vectors to immunologically naive experimental animals almost invariably results in the induction of systemic anti-Ad neutralizing antibodies. To determine if the human systemic humoral host responses to Ad vectors follow a similar pattern, we evaluated the systemic (serum) anti-Ad serotype 5 (Ad5) neutralizing antibodies in humans after administration of first generation (E1(-) E3(-)) Ad5-based gene transfer vectors to different hosts. AdGVCFTR.10 (carrying the normal human cystic fibrosis [CF] transmembrane regulator cDNA) was sprayed (8 x 10(7) to 2 x 10(10) particle units [PU]) repetitively (every 3 months or every 2 weeks) to the airway epithelium of 15 individuals with CF. AdGVCD.10 (carrying the Escherichia coli cytosine deaminase gene) was administered (8 x 10(8) to 8 x 10(9) PU; once a week, twice) directly to liver metastasis of five individuals with colon cancer and by the intradermal route (8 x 10(7) to 8 x 10(9) PU, single administration) to six healthy individuals. AdGVVEGF121.10 (carrying the human vascular endothelial growth factor 121 cDNA) was administered (4 x 10(8) to 4 x 10(9.5) PU, single administration) directly to the myocardium of 11 individuals with ischemic heart disease. Ad vector administration to the airways of individuals with CF evoked no or minimal serum neutralizing antibodies, even with repetitive administration. In contrast, intratumor administration of an Ad vector to individuals with metastatic colon cancer resulted in a robust antibody response, with anti-Ad neutralizing antibody titers of 10(2) to >10(4). Healthy individuals responded to single intradermal Ad vector variably, from induction of no neutralizing anti-Ad antibodies to titers of 5 x 10(3). Likewise, individuals with ischemic heart disease had a variable response to single intramyocardial vector administration, ranging from minimal neutralizing antibody levels to titers of 10(4). Evaluation of the data from all trials showed no correlation between the peak serum neutralizing anti-Ad response and the dose of Ad vector administered (P > 0.1, all comparisons). In contrast, there was a striking correlation between the peak anti-Ad5 neutralizing antibody levels evoked by vector administration and the level of preexisting anti-Ad5 antibodies (P = 0.0001). Thus, unlike the case for experimental animals, administration of Ad vectors to humans does not invariably evoke a systemic anti-Ad neutralizing antibody response. In humans, the extent of the response is dictated by preexisting antibody titers and modified by route of administration but is not dose dependent. Since the extent of anti-Ad neutralizing antibodies will likely modify the efficacy of administration of Ad vectors, these observations are of fundamental importance in designing human gene therapy trials and in interpreting the efficacy of Ad vector-mediated gene transfer.
Figures



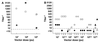
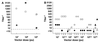
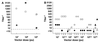

Similar articles
-
Systemic interleukin-6 responses following administration of adenovirus gene transfer vectors to humans by different routes.Mol Ther. 2002 Aug;6(2):287-97. doi: 10.1006/mthe.2002.0658. Mol Ther. 2002. PMID: 12349828 Clinical Trial.
-
Safety of local delivery of low- and intermediate-dose adenovirus gene transfer vectors to individuals with a spectrum of morbid conditions.Hum Gene Ther. 2002 Jan 1;13(1):15-63. doi: 10.1089/10430340152712638. Hum Gene Ther. 2002. PMID: 11779412 Clinical Trial.
-
Analysis of risk factors for local delivery of low- and intermediate-dose adenovirus gene transfer vectors to individuals with a spectrum of comorbid conditions.Hum Gene Ther. 2002 Jan 1;13(1):65-100. doi: 10.1089/10430340152712647. Hum Gene Ther. 2002. PMID: 11779413 Clinical Trial.
-
Gene therapy in cystic fibrosis.Chest. 2001 Sep;120(3 Suppl):124S-131S. doi: 10.1378/chest.120.3_suppl.124s. Chest. 2001. PMID: 11555567 Review.
-
Adenovirus vectors composed of subgroup B adenoviruses.Curr Gene Ther. 2007 Aug;7(4):229-38. doi: 10.2174/156652307781369137. Curr Gene Ther. 2007. PMID: 17969556 Review.
Cited by
-
Vectors for gene therapy of cardiovascular disease.Curr Cardiol Rep. 2000 Jan;2(1):39-47. doi: 10.1007/s11886-000-0024-3. Curr Cardiol Rep. 2000. PMID: 10980871 Review.
-
Neoceptor concept based on molecular complementarity in GPCRs: a mutant adenosine A(3) receptor with selectively enhanced affinity for amine-modified nucleosides.J Med Chem. 2001 Nov 22;44(24):4125-36. doi: 10.1021/jm010232o. J Med Chem. 2001. PMID: 11708915 Free PMC article.
-
A preliminary and comparative evaluation of a novel Ad5 [E1-, E2b-] recombinant-based vaccine used to induce cell mediated immune responses.Immunol Lett. 2009 Jan 29;122(1):44-51. doi: 10.1016/j.imlet.2008.11.003. Epub 2008 Dec 13. Immunol Lett. 2009. PMID: 19073216 Free PMC article.
-
Gene therapy for cystic fibrosis: Challenges and prospects.Front Pharmacol. 2022 Oct 11;13:1015926. doi: 10.3389/fphar.2022.1015926. eCollection 2022. Front Pharmacol. 2022. PMID: 36304167 Free PMC article. Review.
-
Towards fibroid gene therapy: adenovirus-mediated delivery of herpes simplex virus 1 thymidine kinase gene/ganciclovir shrinks uterine leiomyoma in the Eker rat model.Gynecol Obstet Invest. 2009;68(1):19-32. doi: 10.1159/000209675. Epub 2009 Mar 27. Gynecol Obstet Invest. 2009. PMID: 19325244 Free PMC article.
References
-
- Akli S, Caillaud C, Vigne E, Stratford-Perricaudet L D, Poenaru L, Perricaudet M, Kahn A, Peschanski M R. Transfer of a foreign gene into the brain using adenovirus vectors. Nat Genet. 1993;3:224–228. - PubMed
-
- Alavi J B, Judy K, Alavi A, Hackney D, Phillips P, Smith J, Recio A, Wilson J, Eck S. Abstracts of the 1st Annual Meeting of the American Society of Gene Therapy. Thorofare, N.J: American Society of Gene Therapy; 1998. Phase I trial of gene therapy in primary brain tumors; p. 112a.
-
- Anonymous. Human gene marker/therapy clinical protocols. Hum Gene Ther. 1998;9:2805–2852. - PubMed
-
- Bajocchi G, Feldman S H, Crystal R G, Mastrangeli A. Direct in vivo gene transfer to ependymal cells in the central nervous system using recombinant adenovirus vectors. Nat Genet. 1993;3:229–234. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

