Modified VP22 localizes to the cell nucleus during synchronized herpes simplex virus type 1 infection
- PMID: 10400775
- PMCID: PMC112762
- DOI: 10.1128/JVI.73.8.6769-6781.1999
Modified VP22 localizes to the cell nucleus during synchronized herpes simplex virus type 1 infection
Abstract
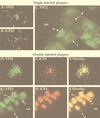
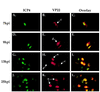
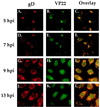
The UL49 gene product (VP22) of herpes simplex virus types 1 and 2 (HSV-1 and HSV-2) is a virion phosphoprotein which accumulates inside infected cells at late stages of infection. We previously (J. A. Blaho, C. Mitchell, and B. Roizman, J. Biol. Chem. 269:17401-17410, 1994) discovered that the form of VP22 packaged into infectious virions differed from VP22 extracted from infected-cell nuclei in that the virion-associated form had a higher electrophoretic mobility in denaturing gels. Based on these results, we proposed that VP22 in virions was "undermodified" in some way. The goal of this study is to document the biological and biochemical properties of VP22 throughout the entire course of a productive HSV-1 infection. We now report the following. (i) VP22 found in infected cells is distributed in at least three distinct subcellular localizations, which we define as cytoplasmic, diffuse, and nuclear, as measured by indirect immunofluorescence. (ii) Using a synchronized infection system, we determined that VP22 exists predominantly in the cytoplasm early in infection and accumulates in the nucleus late in infection. (iii) While cytoplasmic VP22 colocalizes with the HSV-1 glycoprotein D early in infection, the nuclear form of VP22 is not restricted to replication compartments which accumulate ICP4. (iv) VP22 migrates as at least three unique electrophoretic species in denaturing sodium dodecyl sulfate-DATD-polyacrylamide gels. VP22a, VP22b, and VP22c have high, intermediate, and low mobility, respectively. (v) The relative distribution of the various forms of VP22 derived from infected whole-cell extracts varies during the course of infection such that low-mobility species predominate at early times and high-mobility forms accumulate later. (vi) The highest-mobility forms of VP22 partition with the cytoplasmic fraction of infected cells, while the lowest-mobility forms are associated with the nuclear fraction. (vii) Finally, full-length VP22 which partitions in the nucleus incorporates radiolabel from [32P]orthophosphate whereas cytoplasmic VP22 does not. Based on these results, we conclude that modification of VP22 coincides with its appearance in the nucleus during the course of productive HSV-1 infection.
Figures



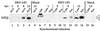

Similar articles
-
Assembly of infectious Herpes simplex virus type 1 virions in the absence of full-length VP22.J Virol. 2000 Nov;74(21):10041-54. doi: 10.1128/jvi.74.21.10041-10054.2000. J Virol. 2000. PMID: 11024133 Free PMC article.
-
Microtubule reorganization during herpes simplex virus type 1 infection facilitates the nuclear localization of VP22, a major virion tegument protein.J Virol. 2001 Sep;75(18):8697-711. doi: 10.1128/jvi.75.18.8697-8711.2001. J Virol. 2001. PMID: 11507215 Free PMC article.
-
Assessment of the subcellular localization of the herpes simplex virus structural protein VP22 in the absence of other viral gene products.Virus Res. 2001 Dec 4;81(1-2):57-68. doi: 10.1016/s0168-1702(01)00355-0. Virus Res. 2001. PMID: 11682125
-
The major tegument structural protein VP22 targets areas of dispersed nucleolin and marginalized chromatin during productive herpes simplex virus 1 infection.Virus Res. 2008 Sep;136(1-2):175-88. doi: 10.1016/j.virusres.2008.05.010. Epub 2008 Jun 12. Virus Res. 2008. PMID: 18584907 Free PMC article.
-
Why does the herpes simplex 1 virus-encoded UL49.5 protein fail to inhibit the TAP-dependent antigen presentation?Biochim Biophys Acta Biomembr. 2023 Dec;1865(8):184200. doi: 10.1016/j.bbamem.2023.184200. Epub 2023 Jul 29. Biochim Biophys Acta Biomembr. 2023. PMID: 37517559 Review.
Cited by
-
Bovine herpesvirus 1 tegument protein VP22 interacts with histones, and the carboxyl terminus of VP22 is required for nuclear localization.J Virol. 2001 Sep;75(17):8251-8. doi: 10.1128/jvi.75.17.8251-8258.2001. J Virol. 2001. PMID: 11483770 Free PMC article.
-
Evaluation of VP22 spread in tissue culture.J Virol. 2000 Jan;74(2):1051-6. doi: 10.1128/jvi.74.2.1051-1056.2000. J Virol. 2000. PMID: 10623773 Free PMC article.
-
Sequential localization of two herpes simplex virus tegument proteins to punctate nuclear dots adjacent to ICP0 domains.J Virol. 2002 Oct;76(20):10365-73. doi: 10.1128/jvi.76.20.10365-10373.2002. J Virol. 2002. PMID: 12239313 Free PMC article.
-
Phosphorylation of the herpes simplex virus tegument protein VP22 has no effect on incorporation of VP22 into the virus but is involved in optimal expression and virion packaging of ICP0.J Virol. 2005 Nov;79(22):14057-68. doi: 10.1128/JVI.79.22.14057-14068.2005. J Virol. 2005. PMID: 16254340 Free PMC article.
-
Single-cell RNA-sequencing of herpes simplex virus 1-infected cells connects NRF2 activation to an antiviral program.Nat Commun. 2019 Oct 25;10(1):4878. doi: 10.1038/s41467-019-12894-z. Nat Commun. 2019. PMID: 31653857 Free PMC article.
References
-
- Barker D E, Roizman B. Identification of three genes nonessential for growth in cell culture near the right terminus of the unique sequences of long component of herpes simplex virus 1. Virology. 1990;177:684–691. - PubMed
-
- Blaho J A, Mitchell C, Roizman B. An amino acid sequence shared by the herpes simplex virus 1 alpha regulatory proteins 0, 4, 22, and 27 predicts the nucleotidylylation of the UL21, UL31, UL47, and UL49 gene products. J Biol Chem. 1994;269:17401–17410. - PubMed
-
- Blaho J A, Roizman B. Analyses of HSV proteins for posttranslational modifications and enzyme functions. In: Brown S M, Maclean A R, editors. Methods in molecular medicine: herpes simplex virus protocols. Vol. 10. Totowa, N.J: Humana Press Inc.; 1998. pp. 237–256. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

