The murine cytomegalovirus chemokine homolog, m131/129, is a determinant of viral pathogenicity
- PMID: 10400778
- PMCID: PMC112765
- DOI: 10.1128/JVI.73.8.6800-6809.1999
The murine cytomegalovirus chemokine homolog, m131/129, is a determinant of viral pathogenicity
Abstract
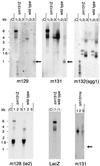
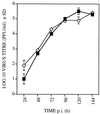
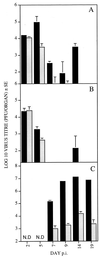
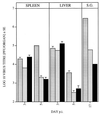
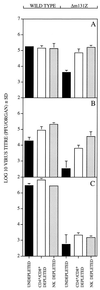
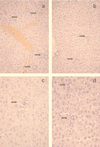
Chemokines are important mediators of the early inflammatory response to infection and modify a wide range of host immune responses. Functional homologs of cellular chemokines have been identified in a number of herpesviruses, suggesting that the subversion of the host chemokine response contributes to the pathogenesis of these viruses. Transcriptional and reverse transcription-PCR analyses demonstrated that the murine cytomegalovirus (MCMV) chemokine homolog, m131, was spliced at the 3' end to the adjacent downstream open reading frame, m129, resulting in a predicted product of 31 kDa, which is significantly larger than most known chemokines. The in vivo impact of m131/129 was investigated by comparing the replication of MCMV mutants having m131/129 deleted (Deltam131/129) with that of wild-type (wt) MCMV. Our studies demonstrate that both wt and Deltam131/129 viruses replicated to equivalent levels during the first 2 to 3 days following in vivo infection. However, histological studies demonstrated that the early inflammatory response elicited by Deltam131/129 was reduced compared with that of wt MCMV. Furthermore, the Deltam131/129 mutants failed to establish a high-titer infection in the salivary glands. These results suggest that m131/129 possesses proinflammatory properties in vivo and is important for the dissemination of MCMV to or infection of the salivary gland. Notably, the Deltam131/129 mutants were cleared more rapidly from the spleen and liver during acute infection compared with wt MCMV. The accelerated clearance of the mutants was dependent on NK cells and cells of the CD4(+) CD8(+) phenotype. These data suggest that m131/129 may also contribute to virus mechanisms of immune system evasion during early infection, possibly through the interference of NK cells and T cells.
Figures


Similar articles
-
The r131 gene of rat cytomegalovirus encodes a proinflammatory CC chemokine homolog which is essential for the production of infectious virus in the salivary glands.Virus Genes. 2004 Aug;29(1):43-61. doi: 10.1023/B:VIRU.0000032788.53592.7c. Virus Genes. 2004. PMID: 15215683
-
Murine cytomegalovirus CC chemokine homolog MCK-2 (m131-129) is a determinant of dissemination that increases inflammation at initial sites of infection.J Virol. 2001 Oct;75(20):9966-76. doi: 10.1128/JVI.75.20.9966-9976.2001. J Virol. 2001. PMID: 11559829 Free PMC article.
-
Fatal attraction: cytomegalovirus-encoded chemokine homologs.Curr Top Microbiol Immunol. 2002;269:235-56. doi: 10.1007/978-3-642-59421-2_14. Curr Top Microbiol Immunol. 2002. PMID: 12224512
-
MCMV avoidance of recognition and control by NK cells.Semin Immunopathol. 2014 Nov;36(6):641-50. doi: 10.1007/s00281-014-0441-9. Epub 2014 Aug 21. Semin Immunopathol. 2014. PMID: 25141793 Review.
-
The salivary glands as a privileged site of cytomegalovirus immune evasion and persistence.Med Microbiol Immunol. 2008 Jun;197(2):205-13. doi: 10.1007/s00430-008-0077-2. Epub 2008 Feb 8. Med Microbiol Immunol. 2008. PMID: 18259775 Review.
Cited by
-
Human herpesvirus 8 (HHV-8)-encoded cytokines induce expression of and autocrine signaling by vascular endothelial growth factor (VEGF) in HHV-8-infected primary-effusion lymphoma cell lines and mediate VEGF-independent antiapoptotic effects.J Virol. 2001 Nov;75(22):10933-40. doi: 10.1128/JVI.75.22.10933-10940.2001. J Virol. 2001. PMID: 11602733 Free PMC article.
-
Murine Cytomegalovirus Glycoprotein O Promotes Epithelial Cell Infection In Vivo.J Virol. 2019 Jan 17;93(3):e01378-18. doi: 10.1128/JVI.01378-18. Print 2019 Feb 1. J Virol. 2019. PMID: 30404805 Free PMC article.
-
Two glycosaminoglycan-binding domains of the mouse cytomegalovirus-encoded chemokine MCK-2 are critical for oligomerization of the full-length protein.J Biol Chem. 2017 Jun 9;292(23):9613-9626. doi: 10.1074/jbc.M117.785121. Epub 2017 Apr 21. J Biol Chem. 2017. PMID: 28432120 Free PMC article.
-
Cytomegalovirus restricts ICOSL expression on antigen-presenting cells disabling T cell co-stimulation and contributing to immune evasion.Elife. 2021 Jan 18;10:e59350. doi: 10.7554/eLife.59350. Elife. 2021. PMID: 33459589 Free PMC article.
-
Functional characterization of chimpanzee cytomegalovirus chemokine, vCXCL-1(CCMV).Virology. 2007 Aug 1;364(2):454-65. doi: 10.1016/j.virol.2007.03.002. Epub 2007 Apr 11. Virology. 2007. PMID: 17433398 Free PMC article.
References
-
- Ahuja S K, Murphy P M. Molecular piracy of mammalian interleukin 8 receptor type B by herpesvirus saimiri. J Biol Chem. 1993;268:20691–20694. - PubMed
-
- Alcami A, Symons J A, Collins P D, Williams T J, Smith G L. Blockade of chemokine activity by a soluble chemokine binding protein from vaccinia virus. J Immunol. 1998;160:624–633. - PubMed
-
- Arvanitakis L, Geras-Raaka E, Varma A, Gershengorn M C, Cesarman E. Human herpesvirus KSHV encodes a constitutively active G protein-coupled receptor linked to cell proliferation. Nature. 1997;385:347–350. - PubMed
-
- Bazan, J. F., K. B. Bacon, G. Hardiman, W. Wang, K. Soo, D. Rossi, D. R. Greaves, A. Zlotnik, and T. J. Schall. A new class of membrane-bound chemokine with a CX3C motif. Nature 385:640–644. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

