In vivo monocyte tropism of pathogenic feline immunodeficiency viruses
- PMID: 10400783
- PMCID: PMC112770
- DOI: 10.1128/JVI.73.8.6852-6861.1999
In vivo monocyte tropism of pathogenic feline immunodeficiency viruses
Abstract
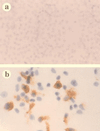
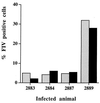
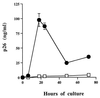
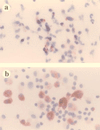
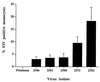
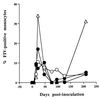
Virus-infected monocytes rarely are detected in the bloodstreams of animals or people infected with immunodeficiency-inducing lentiviruses, yet tissue macrophages are thought to be a major reservoir of virus-infected cells in vivo. We have identified feline immunodeficiency virus (FIV) clinical isolates that are pathogenic in cats and readily transmitted vertically. We report here that five of these FIV isolates are highly monocytotropic in vivo. However, while FIV-infected monocytes were numerous in the blood of experimentally infected cats, viral antigen was not detectable in freshly isolated cells. Only after a short-term (at least 12-h) in vitro monocyte culture were FIV antigens detectable (by immunocytochemical analysis or enzyme-linked immunosorbent assay). In vitro experiments suggested that monocyte adherence provided an important trigger for virus antigen expression. In the blood of cats infected with a prototype monocytotropic isolate (FIV subtype B strain 2542), infected monocytes appeared within 2 weeks, correlating with high blood mononuclear-cell-associated viral titers and CD4 cell depletion. By contrast, infected monocytes could not be detected in the blood of cats infected with a less pathogenic FIV strain (FIV subtype A strain Petaluma). We concluded that some strains of FIV are monocytotropic in vivo. Moreover, this property may relate to virus virulence, vertical transmission, and infection of tissue macrophages.
Figures




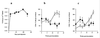
Similar articles
-
In vivo lymphocyte tropism of feline immunodeficiency virus.J Virol. 1993 Sep;67(9):5175-86. doi: 10.1128/JVI.67.9.5175-5186.1993. J Virol. 1993. PMID: 7688819 Free PMC article.
-
Effects of feline immunodeficiency virus on feline monocyte-derived dendritic cells infected by spinoculation.J Gen Virol. 2007 Sep;88(Pt 9):2574-2582. doi: 10.1099/vir.0.82926-0. J Gen Virol. 2007. PMID: 17698669
-
Virulence differences between two field isolates of feline immunodeficiency virus (FIV-APetaluma and FIV-CPGammar) in young adult specific pathogen free cats.Vet Immunol Immunopathol. 2001 May 10;79(1-2):53-67. doi: 10.1016/s0165-2427(01)00252-5. Vet Immunol Immunopathol. 2001. PMID: 11356250
-
Feline immunodeficiency virus: an interesting model for AIDS studies and an important cat pathogen.Clin Microbiol Rev. 1995 Jan;8(1):87-112. doi: 10.1128/CMR.8.1.87. Clin Microbiol Rev. 1995. PMID: 7704896 Free PMC article. Review.
-
Feline immunodeficiency virus infection.Vet Immunol Immunopathol. 1989 May;21(1):111-29. doi: 10.1016/0165-2427(89)90134-7. Vet Immunol Immunopathol. 1989. PMID: 2549690 Review.
Cited by
-
FIV establishes a latent infection in feline peripheral blood CD4+ T lymphocytes in vivo during the asymptomatic phase of infection.Retrovirology. 2012 Feb 7;9:12. doi: 10.1186/1742-4690-9-12. Retrovirology. 2012. PMID: 22314004 Free PMC article.
-
Immunopathogenesis of feline immunodeficiency virus infection in the fetal and neonatal cat.Front Biosci. 2007 May 1;12:3668-82. doi: 10.2741/2343. Front Biosci. 2007. PMID: 17485330 Free PMC article. Review.
-
In vitro activation of feline immunodeficiency virus in ramified microglial cells from asymptomatically infected cats.J Virol. 2001 Sep;75(17):8090-5. doi: 10.1128/jvi.75.17.8090-8095.2001. J Virol. 2001. PMID: 11483754 Free PMC article.
-
The neuropathogenesis of feline immunodeficiency virus infection: barriers to overcome.Vet J. 2011 Jun;188(3):260-9. doi: 10.1016/j.tvjl.2010.03.022. Epub 2010 Apr 24. Vet J. 2011. PMID: 20418131 Free PMC article. Review.
-
Suppression of immunodeficiency virus-associated neural damage by the p75 neurotrophin receptor ligand, LM11A-31, in an in vitro feline model.J Neuroimmune Pharmacol. 2012 Jun;7(2):388-400. doi: 10.1007/s11481-011-9325-0. Epub 2011 Dec 10. J Neuroimmune Pharmacol. 2012. PMID: 22161560 Free PMC article.
References
-
- Alkhatib F, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. CC CKR5: a RANTES, MIP-I‡, MIP-1 · receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. - PubMed
-
- Bagasra O, Hauptman S P, Lischner H W, Sachs M, Pomerantz R J. Detection of human immunodeficiency virus type 1 provirus in mononuclear cells by in situ polymerase chain reaction. N Engl J Med. 1992;326:1385–1391. - PubMed
-
- Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath P D, Wu L, Mackay C R, LaRosa G, Newman W, Gerard N, Gerard C. The · -chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85:1135–1148. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

