Human MxA protein protects mice lacking a functional alpha/beta interferon system against La crosse virus and other lethal viral infections
- PMID: 10400797
- PMCID: PMC112784
- DOI: 10.1128/JVI.73.8.6984-6991.1999
Human MxA protein protects mice lacking a functional alpha/beta interferon system against La crosse virus and other lethal viral infections
Abstract
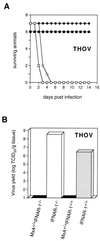
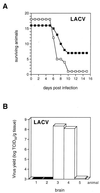
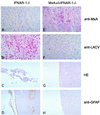
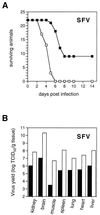
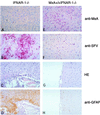
The human MxA protein is part of the antiviral state induced by alpha/beta interferon (IFN-alpha/beta). MxA inhibits the multiplication of several RNA viruses in cell culture. However, its antiviral potential in vivo has not yet been fully explored. We have generated MxA-transgenic mice that lack a functional IFN system by crossing MxA-transgenic mice constitutively expressing MxA with genetically targeted (knockout) mice lacking the beta subunit of the IFN-alpha/beta receptor (IFNAR-1(-/-) mice). These mice are an ideal animal model to investigate the unique antiviral activity of human MxA in vivo, because they are unable to express other IFN-induced proteins. Here, we show that MxA confers resistance to Thogoto virus, La Crosse virus, and Semliki Forest virus. No Thogoto virus progeny was detectable in MxA-transgenic mice, indicating an efficient block of virus replication at the primary site of infection. In the case of La Crosse virus, MxA restricted invasion of the central nervous system. In contrast, Semliki Forest virus multiplication in the brain was detectable in both MxA-expressing and nonexpressing IFNAR-1(-/-) mice. However, viral titers were clearly reduced in MxA-transgenic mice. Our results demonstrate that MxA does not need the help of other IFN-induced proteins for activity but is a powerful antiviral agent on its own. Moreover, the results suggest that MxA may protect humans from potential fatal infections by La Crosse virus and other viral pathogens.
Figures
Similar articles
-
[Alpha interferon, antiviral proteins and their value in clinical medicine].Ann Biol Clin (Paris). 1999 Nov-Dec;57(6):659-66. Ann Biol Clin (Paris). 1999. PMID: 10572214 Review. French.
-
Human MxA protein confers resistance to Semliki Forest virus and inhibits the amplification of a Semliki Forest virus-based replicon in the absence of viral structural proteins.J Virol. 1998 Feb;72(2):1516-22. doi: 10.1128/JVI.72.2.1516-1522.1998. J Virol. 1998. PMID: 9445055 Free PMC article.
-
Enhanced virus resistance of transgenic mice expressing the human MxA protein.J Virol. 1995 Jul;69(7):4506-10. doi: 10.1128/JVI.69.7.4506-4510.1995. J Virol. 1995. PMID: 7769712 Free PMC article.
-
Role of nucleotide binding and GTPase domain dimerization in dynamin-like myxovirus resistance protein A for GTPase activation and antiviral activity.J Biol Chem. 2015 May 15;290(20):12779-92. doi: 10.1074/jbc.M115.650325. Epub 2015 Mar 31. J Biol Chem. 2015. PMID: 25829498 Free PMC article.
-
Interferons, Mx genes, and resistance to influenza virus.Am J Respir Crit Care Med. 1995 Oct;152(4 Pt 2):S67-71. doi: 10.1164/ajrccm/152.4_Pt_2.S67. Am J Respir Crit Care Med. 1995. PMID: 7551417 Review.
Cited by
-
Innate immune response to La Crosse virus infection.J Neurovirol. 2014 Apr;20(2):150-6. doi: 10.1007/s13365-013-0186-6. Epub 2013 Jul 12. J Neurovirol. 2014. PMID: 23846288 Review.
-
Role of Innate Interferon Responses at the Ocular Surface in Herpes Simplex Virus-1-Induced Herpetic Stromal Keratitis.Pathogens. 2023 Mar 10;12(3):437. doi: 10.3390/pathogens12030437. Pathogens. 2023. PMID: 36986359 Free PMC article. Review.
-
Throw out the Map: Neuropathogenesis of the Globally Expanding California Serogroup of Orthobunyaviruses.Viruses. 2019 Aug 29;11(9):794. doi: 10.3390/v11090794. Viruses. 2019. PMID: 31470541 Free PMC article. Review.
-
Searching for interferon-induced genes that inhibit hepatitis B virus replication in transgenic mouse hepatocytes.J Virol. 2003 Jan;77(2):1227-36. doi: 10.1128/jvi.77.2.1227-1236.2003. J Virol. 2003. PMID: 12502840 Free PMC article.
-
Influenza A virus strains differ in sensitivity to the antiviral action of Mx-GTPase.J Virol. 2008 Apr;82(7):3624-31. doi: 10.1128/JVI.01753-07. Epub 2008 Jan 16. J Virol. 2008. PMID: 18199636 Free PMC article.
References
-
- Albanese M, Bruno-Smiraglia C, Di Cuonzo G, Lavagnino A, Srihongse S. Isolation of Thogoto virus from Rhipicephalus bursa ticks in western Sicily. Acta Virol. 1972;16:267. - PubMed
-
- Arnheiter H, Frese M, Kamadur R, Meier E, Haller O. Mx transgenic mice—animal models of health. Curr Top Microbiol Immunol. 1995;206:119–147. - PubMed
-
- Centers for Disease Control and Prevention. Arboviral infections of the central nervous system—United States, 1996–1997. Morbid Mortal Weekly Rep. 1998;47:517–522. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

