Comparative effects of cyclo-oxygenase and nitric oxide synthase inhibition on the development and reversal of spinal opioid tolerance
- PMID: 10401553
- PMCID: PMC1566057
- DOI: 10.1038/sj.bjp.0702587
Comparative effects of cyclo-oxygenase and nitric oxide synthase inhibition on the development and reversal of spinal opioid tolerance
Abstract
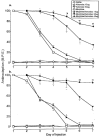
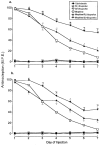
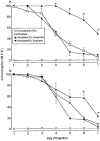
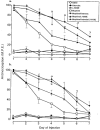
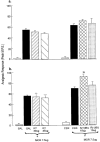
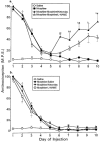
1. This study examined the effects of the COX inhibitors, ketorolac and ibuprofen, and the NOS inhibitor L-NAME for their potential to both inhibit the development and reverse tolerance to the antinociceptive action of morphine. 2. Repeated administration of intrathecal morphine (15 micrograms), once daily, resulted in a progressive decline of antinociceptive effect and an increase in the ED50 value in the tailflick and paw pressure tests. Co-administration of ketorolac (30 and 45 micrograms) or S(+) ibuprofen (10 micrograms) with morphine (15 micrograms) prevented the decline of antinociceptive effect and increase in ED50 value. Similar treatment with L-NAME (100 micrograms) exerted weaker effects. Administration of S(+) but not R(-) ibuprofen (10 mg kg-1) had similar effects on systemic administration of morphine (15 mg kg-1). 3. Intrathecal or systemic administration of the COX or NOS inhibitors did not alter the baseline responses in either tests. Acute keterolac or S(+) ibuprofen also did not potentiate the acute actions of spinal or systemic morphine, but chronic intrathecal administration of these agents increased the potency of acute morphine. 4. In animals already tolerant to intrathecal morphine, subsequent administration of ketorolac (30 micrograms) with morphine (15 micrograms) partially restored the antinociceptive effect and ED50 value of acute morphine, reflecting the reversal of tolerance. Intrathecal L-NAME (100 micrograms) exerted a weaker effect. 5. These data suggest that spinal COX activity, and to a lesser extent NOS activity, contributes to the development and expression of opioid tolerance. Inhibition of COX may represent a useful approach for the prevention as well as reversal of opioid tolerance.
Figures
References
-
- ADAMS S.S., BRESLOFF P., MASON C.G. Pharmacological differences between the optical isomers of ibuprofen: evidence for metabolic inversion of the (−)-isomer. J. Pharm. Pharmacol. 1976;28:256–257. - PubMed
-
- AMMER H., SCHULZ R. Morphine dependence in human neuroblastoma SH-SY5Y cells is associated with adaptive changes in both the quantity and functional interaction of PGE1 receptors and stimulatory G proteins. Brain Res. 1996;707:235–244. - PubMed
-
- BONEBERG E.M., ZOU M.H., ULLRICH V. Inhibition of cyclooxygenase-1 and -2 by R(−)- and S(+)-ibuprofen. J. Clin. Pharmacol. 1996;36 Suppl:19S. - PubMed
-
- CRYER B., FELDMAN M. Cyclooxygenase-1 and cyclooxygenase-2 selectivity of widely used nonsteroidal anti-inflammatory drugs. Am. J. Med. 1998;104:413–421. - PubMed
-
- D'AMOUR F.E., SMITH D.L. A method for determining loss of pain sensation. J. Pharmacol. Exp. Ther. 1941;72:74–79.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

