A role for the vesicle tethering protein, p115, in the post-mitotic stacking of reassembling Golgi cisternae in a cell-free system
- PMID: 10402460
- PMCID: PMC2199741
- DOI: 10.1083/jcb.146.1.57
A role for the vesicle tethering protein, p115, in the post-mitotic stacking of reassembling Golgi cisternae in a cell-free system
Abstract
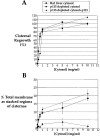
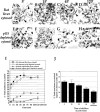
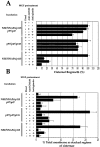
During telophase, Golgi cisternae are regenerated and stacked from a heterogeneous population of tubulovesicular clusters. A cell-free system that reconstructs these events has revealed that cisternal regrowth requires interplay between soluble factors and soluble N-ethylmaleimide (NEM)-sensitive fusion protein (NSF) attachment protein receptors (SNAREs) via two intersecting pathways controlled by the ATPases, p97 and NSF. Golgi reassembly stacking protein 65 (GRASP65), an NEM-sensitive membrane-bound component, is required for the stacking process. NSF-mediated cisternal regrowth requires a vesicle tethering protein, p115, which we now show operates through its two Golgi receptors, GM130 and giantin. p97-mediated cisternal regrowth is p115-independent, but we now demonstrate a role for p115, in conjunction with its receptors, in stacking p97 generated cisternae. Temporal analysis suggests that p115 plays a transient role in stacking that may be upstream of GRASP65-mediated stacking. These results implicate p115 and its receptors in the initial alignment and docking of single cisternae that may be an important prerequisite for stack formation.
Figures



References
-
- Acharya U., Jacobs R., Peters J.M., Watson N., Farquhar M.G., Malhotra V. The formation of Golgi stacks from vesiculated Golgi membranes requires two distinct fusion events. Cell. 1995;82:895–904. - PubMed
-
- Acharya U., Mallabiabarrena A., Acharya J.K., Malhotra V. Signaling via mitogen-activated protein-kinase (MEK 1) is required for Golgi fragmentation during mitosis. Cell. 1998;92:183–192. - PubMed
-
- Barr F.A., Puype M., Vandekerckhove J., Warren G. GRASP65, a protein involved in the stacking of Golgi cisternae. Cell. 1997;91:253–262. - PubMed

