DAP-kinase participates in TNF-alpha- and Fas-induced apoptosis and its function requires the death domain
- PMID: 10402466
- PMCID: PMC2199731
- DOI: 10.1083/jcb.146.1.141
DAP-kinase participates in TNF-alpha- and Fas-induced apoptosis and its function requires the death domain
Abstract
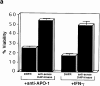
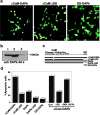
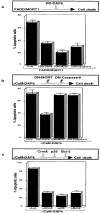
Death-associated protein (DAP)-kinase is a calcium/calmodulin regulated serine/threonine kinase that carries ankyrin repeats, a death domain, and is localized to the cytoskeleton. Here, we report that this kinase is involved in tumor necrosis factor (TNF)-alpha and Fas-induced apoptosis. Expression of DAP-kinase antisense RNA protected cells from killing by anti-Fas/APO-1 agonistic antibodies. Deletion of the death domain abrogated the apoptotic functions of the kinase, thus, documenting for the first time the importance of this protein domain. Overexpression of a fragment encompassing the death domain of DAP-kinase acted as a specific dominant negative mutant that protected cells from TNF-alpha, Fas, and FADD/MORT1-induced cell death. DAP-kinase apoptotic function was blocked by bcl-2 as well as by crmA and p35 inhibitors of caspases, but not by the dominant negative mutants of FADD/MORT1 or of caspase 8. Thus, it functions downstream to the receptor complex and upstream to other caspases. The multidomain structure of this serine/threonine kinase, combined with its involvement in cell death induced by several different triggers, place DAP-kinase at one of the central molecular pathways leading to apoptosis.
Figures


References
-
- Beidler D.R., Tewari M., Friesen P.D., Poirier G., Dixit V.M. The baculovirus p35 protein inhibits Fas- and tumor necrosis factor–induced apoptosis. J. Biol. Chem. 1995;270:16526–16528. - PubMed
-
- Boldin M.P., Varfolomeev E.E., Pancer Z., Mett I.L., Camonis J.H., Wallach D. A novel protein that interacts with the death domain of Fas/APO1 contains a sequence motif related to the death domain. J. Biol. Chem. 1995;270:7795–7798. - PubMed
-
- Boldin M.P., Goncharov T.M., Goltsev Y.V., Wallach D. Involvement of MACH, a novel Mort1/FADD–interacting protease, in Fas/APO-1 and TNF receptor-induced cell death. Cell. 1996;85:803–815. - PubMed
-
- Chinnaiyan A.M., O'Rourke K., Tewari M., Dixit V.M. FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell. 1995;81:505–512. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

