Genomic organization, expression, and analysis of the troponin C gene pat-10 of Caenorhabditis elegans
- PMID: 10402470
- PMCID: PMC2199735
- DOI: 10.1083/jcb.146.1.193
Genomic organization, expression, and analysis of the troponin C gene pat-10 of Caenorhabditis elegans
Abstract
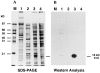
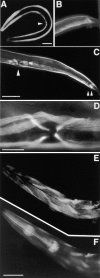
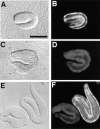
We have cloned and characterized the troponin C gene, pat-10 of the nematode Caenorhabditis elegans. At the amino acid level nematode troponin C is most similar to troponin C of Drosophila (45% identity) and cardiac troponin C of vertebrates. Expression studies demonstrate that this troponin is expressed in body wall muscle throughout the life of the animal. Later, vulval muscles and anal muscles also express this troponin C isoform. The structural gene for this troponin is pat-10 and mutations in this gene lead to animals that arrest as twofold paralyzed embryos late in development. We have sequenced two of the mutations in pat-10 and both had identical two mutations in the gene; one changes D64 to N and the other changes W153 to a termination site. The missense alteration affects a calcium-binding site and eliminates calcium binding, whereas the second mutation eliminates binding to troponin I. These combined biochemical and in vivo studies of mutant animals demonstrate that this troponin is essential for proper muscle function during development.
Figures






References
-
- Anderson P. Molecular genetics of nematode muscle. Annu. Rev. Genet. 1990;23:507–525. - PubMed
-
- Barstead R.J., Waterston R.H. The Basal component of the nematode dense-body is binculin. J. Biol. Chem. 1989;264:10177–10185. - PubMed
-
- Blumenthal T., Steward K. RNA processing and gene structure. In: Riddle D.L., editor. C. elegans II. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1997. pp. 117–145. - PubMed
-
- Bullard B., Kevin L., Larkins A., Butcher G., Karlik C., Fyrberg E. Troponin of asynchronous flight muscle. J. Mol. Biol. 1988;204:621–637. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

