alpha-bungarotoxin receptors contain alpha7 subunits in two different disulfide-bonded conformations
- PMID: 10402471
- PMCID: PMC2199736
- DOI: 10.1083/jcb.146.1.203
alpha-bungarotoxin receptors contain alpha7 subunits in two different disulfide-bonded conformations
Abstract
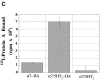
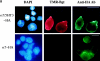
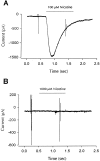
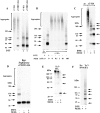
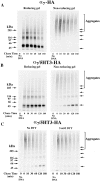
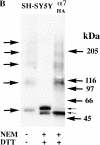
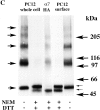
Neuronal nicotinic alpha7 subunits assemble into cell-surface complexes that neither function nor bind alpha-bungarotoxin when expressed in tsA201 cells. Functional alpha-bungarotoxin receptors are expressed if the membrane-spanning and cytoplasmic domains of the alpha7 subunit are replaced by the homologous regions of the serotonin-3 receptor subunit. Bgt-binding surface receptors assembled from chimeric alpha7/serotonin-3 subunits contain subunits in two different conformations as shown by differences in redox state and other features of the subunits. In contrast, alpha7 subunit complexes in the same cell line contain subunits in a single conformation. The appearance of a second alpha7/serotonin-3 subunit conformation coincides with the formation of alpha-bungarotoxin-binding sites and intrasubunit disulfide bonding, apparently within the alpha7 domain of the alpha7/serotonin-3 chimera. In cell lines of neuronal origin that produce functional alpha7 receptors, alpha7 subunits undergo a conformational change similar to alpha7/serotonin-3 subunits. alpha7 subunits, thus, can fold and assemble by two different pathways. Subunits in a single conformation assemble into nonfunctional receptors, or subunits expressed in specialized cells undergo additional processing to produce functional, alpha-bungarotoxin-binding receptors with two alpha7 conformations. Our results suggest that alpha7 subunit diversity can be achieved postranslationally and is required for functional homomeric receptors.
Figures





References
-
- Alkondon M., Albuquerque E.X. Diversity of nicotinic acetylcholine receptors in rat hippocampal neurons. I. Pharmacological and functional evidence for distinct structural subtypes. J. Pharmacol. Exp. Ther. 1993;265:1455–1473. - PubMed
-
- Alkondon M., Rocha E.S., Maelicke A., Albuquerque E.X. Diversity of nicotinic acetylcholine receptors in rat brain. V. Alpha-bungarotoxin-sensitive nicotinic receptors in olfactory bulb neurons and presynaptic modulation of glutamate release. J. Pharmacol. Exp. Ther. 1996;278:1460–1471. - PubMed
-
- Blount P., Merlie J.P. Molecular basis of the two nonequivalent ligand binding sites of the muscle nicotinic acetylcholine receptor. Neuron. 1989;3:349–357. - PubMed

