Recycling of E-cadherin: a potential mechanism for regulating cadherin dynamics
- PMID: 10402472
- PMCID: PMC2199726
Recycling of E-cadherin: a potential mechanism for regulating cadherin dynamics
Abstract
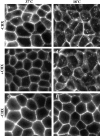
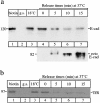
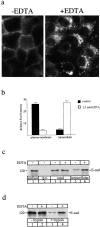
E-Cadherin plays critical roles in many aspects of cell adhesion, epithelial development, and the establishment and maintenance of epithelial polarity. The fate of E-cadherin once it is delivered to the basolateral cell surface, and the mechanisms which govern its participation in adherens junctions, are not well understood. Using surface biotinylation and recycling assays, we observed that some of the cell surface E-cadherin is actively internalized and is then recycled back to the plasma membrane. The pool of E-cadherin undergoing endocytosis and recycling was markedly increased in cells without stable cell-cell contacts, i.e., in preconfluent cells and after cell contacts were disrupted by depletion of extracellular Ca2+, suggesting that endocytic trafficking of E-cadherin is regulated by cell-cell contact. The reformation of cell junctions after replacement of Ca2+ was then found to be inhibited when recycling of endocytosed E-cadherin was disrupted by bafilomycin treatment. The endocytosis and recycling of E-cadherin and of the transferrin receptor were similarly inhibited by potassium depletion and by bafilomycin treatment, and both proteins were accumulated in intracellular compartments by an 18 degrees C temperature block, suggesting that endocytosis may occur via a clathrin-mediated pathway. We conclude that a pool of surface E-cadherin is constantly trafficked through an endocytic, recycling pathway and that this may provide a mechanism for regulating the availability of E-cadherin for junction formation in development, tissue remodeling, and tumorigenesis.
Figures








Similar articles
-
Protein kinase C regulates endocytosis and recycling of E-cadherin.Am J Physiol Cell Physiol. 2002 Aug;283(2):C489-99. doi: 10.1152/ajpcell.00566.2001. Am J Physiol Cell Physiol. 2002. PMID: 12107059
-
Selective degradation of E-cadherin and dissolution of E-cadherin-catenin complexes in epithelial ischemia.Am J Physiol Renal Physiol. 2000 May;278(5):F847-52. doi: 10.1152/ajprenal.2000.278.5.F847. Am J Physiol Renal Physiol. 2000. PMID: 10807598
-
RAC1 regulates adherens junctions through endocytosis of E-cadherin.Mol Biol Cell. 2001 Apr;12(4):847-62. doi: 10.1091/mbc.12.4.847. Mol Biol Cell. 2001. PMID: 11294891 Free PMC article.
-
Adherens Junctions on the Move-Membrane Trafficking of E-Cadherin.Cold Spring Harb Perspect Biol. 2017 Mar 1;9(3):a029140. doi: 10.1101/cshperspect.a029140. Cold Spring Harb Perspect Biol. 2017. PMID: 28096264 Free PMC article. Review.
-
Cadherin tales: Regulation of cadherin function by endocytic membrane trafficking.Traffic. 2016 Dec;17(12):1262-1271. doi: 10.1111/tra.12448. Epub 2016 Oct 27. Traffic. 2016. PMID: 27624909 Review.
Cited by
-
E-Cadherin Expression in Relation to Clinicopathological Parameters and Survival of Patients with Epithelial Ovarian Cancer.Int J Mol Sci. 2022 Nov 19;23(22):14383. doi: 10.3390/ijms232214383. Int J Mol Sci. 2022. PMID: 36430858 Free PMC article. Review.
-
Nano-structure of vitronectin/heparin on cell membrane for stimulating single cell in iPSC-derived embryoid body.iScience. 2021 Mar 11;24(4):102297. doi: 10.1016/j.isci.2021.102297. eCollection 2021 Apr 23. iScience. 2021. PMID: 33851104 Free PMC article.
-
4.1N is involved in a flotillin-1/β-catenin/Wnt pathway and suppresses cell proliferation and migration in non-small cell lung cancer cell lines.Tumour Biol. 2016 Sep;37(9):12713-12723. doi: 10.1007/s13277-016-5146-3. Epub 2016 Jul 22. Tumour Biol. 2016. PMID: 27448302
-
Late domain dependent E-cadherin recruitment into extracellular vesicles.Front Cell Dev Biol. 2022 Sep 7;10:878620. doi: 10.3389/fcell.2022.878620. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36172289 Free PMC article.
-
Adherens junctions as molecular regulators of emergent tissue mechanics.Nat Rev Mol Cell Biol. 2024 Apr;25(4):252-269. doi: 10.1038/s41580-023-00688-7. Epub 2023 Dec 13. Nat Rev Mol Cell Biol. 2024. PMID: 38093099 Review.
References
-
- Alexander J.S., Jackson S.A., Chaney E., Kevil C.G., Haselton F.R. The role of cadherin endocytosis in endothelial barrier regulationinvolvement of protein kinase C and actin-cadherin interactions. Inflammation. 1998;22:419–433. - PubMed
-
- Bradbury N.A., Jilling T., Berta G., Sorscher E.J., Bridges R.J., Kirk K.L. Regulation of plasma membrane recycling by CFTR. Science. 1992;256:530–532. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
