Glypican-3-deficient mice exhibit developmental overgrowth and some of the abnormalities typical of Simpson-Golabi-Behmel syndrome
- PMID: 10402475
- PMCID: PMC2199732
- DOI: 10.1083/jcb.146.1.255
Glypican-3-deficient mice exhibit developmental overgrowth and some of the abnormalities typical of Simpson-Golabi-Behmel syndrome
Abstract
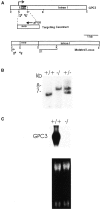
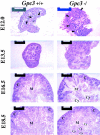
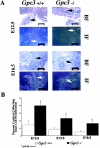
Glypicans are a family of heparan sulfate proteoglycans that are linked to the cell surface through a glycosyl-phosphatidylinositol anchor. One member of this family, glypican-3 (Gpc3), is mutated in patients with the Simpson-Golabi-Behmel syndrome (SGBS). These patients display pre- and postnatal overgrowth, and a varying range of dysmorphisms. The clinical features of SGBS are very similar to the more extensively studied Beckwith-Wiedemann syndrome (BWS). Since BWS has been associated with biallelic expression of insulin-like growth factor II (IGF-II), it has been proposed that GPC3 is a negative regulator of IGF-II. However, there is still no biochemical evidence indicating that GPC3 plays such a role.Here, we report that GPC3-deficient mice exhibit several of the clinical features observed in SGBS patients, including developmental overgrowth, perinatal death, cystic and dyplastic kidneys, and abnormal lung development. A proportion of the mutant mice also display mandibular hypoplasia and an imperforate vagina. In the particular case of the kidney, we demonstrate that there is an early and persistent developmental abnormality of the ureteric bud/collecting system due to increased proliferation of cells in this tissue element. The degree of developmental overgrowth of the GPC3-deficient mice is similar to that of mice deficient in IGF receptor type 2 (IGF2R), a well characterized negative regulator of IGF-II. Unlike the IGF2R-deficient mice, however, the levels of IGF-II in GPC3 knockouts are similar to those of the normal littermates.
Figures





References
-
- Behmel A., Plochl E., Rosenkranz W. A new X-linked dysplasia gigantism syndromeidentical with the Simpson dysplasia syndrome? Hum. Genet. 1984;67:409–413. - PubMed
-
- Bonneh-Barkay D., Shlissel M., Berman B., Shaoul E., Admon A., Vlodavsky I., Carey D.J., Asundi V.K., Reich-Slotky R., Ron D. Identification of glypican as a dual modulator of the biological activity of fibroblast growth factors. J. Biol. Chem. 1997;272:12415–12421. - PubMed
-
- Brown K.W., Villar A.J., Bickmore W., Clayton-Smith J., Catchpoole D., Maher E.R., Reik W. Imprinting mutation in the Beckwith-Wiedemann syndrome leads to biallelic IGF2 expression through an H19-independent pathway. Hum. Mol. Genet. 1996;5:2027–2032. - PubMed
-
- Cunliffe-Beamer T.L., Feldman D.B. Vaginal septa in miceincidence, inheritance, and effect on reproductive performance. Lab. Anim. Sci. 1976;26:895–898. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

